Type 2 diabetes (T2D), formerly known as adult-onset diabetes, is a form of diabetes mellitus that is characterized by high blood sugar, insulin resistance, and relative lack of insulin. Common symptoms include increased thirst, frequent urination, fatigue and unexplained weight loss. Symptoms may also include increased hunger, having a sensation of pins and needles, and sores (wounds) that do not heal. Often symptoms come on slowly. Long-term complications from high blood sugar include heart disease, strokes, diabetic retinopathy which can result in blindness, kidney failure, and poor blood flow in the limbs which may lead to amputations. The sudden onset of hyperosmolar hyperglycemic state may occur; however, ketoacidosis is uncommon.
Metformin, sold under the brand name Glucophage, among others, is the main first-line medication for the treatment of type 2 diabetes, particularly in people who are overweight. It is also used in the treatment of polycystic ovary syndrome. It is sometimes used as an off-label adjunct to lessen the risk of metabolic syndrome in people who take antipsychotics. Metformin is not associated with weight gain and is taken by mouth.
Drugs used in diabetes treat diabetes mellitus by decreasing the glucose level in the blood. With the exception of insulin, most GLP receptor agonists, and pramlintide, all are administered orally and are thus also called oral hypoglycemic agents or oral antihyperglycemic agents. There are different classes of hypoglycemic drugs, and their selection depends on the nature of diabetes, age, and situation of the person, as well as other factors.

Pioglitazone, sold under the brand name Actos among others, is an anti-diabetic medication used to treat type 2 diabetes. It may be used with metformin, a sulfonylurea, or insulin. Use is recommended together with exercise and diet. It is not recommended in type 1 diabetes. It is taken by mouth.
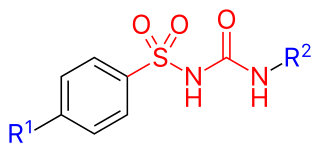
Sulfonylureas or sulphonylureas are a class of organic compounds used in medicine and agriculture. The functional group consists of a sulfonyl group (-S(=O)2) with its sulphur atom bonded to a nitrogen atom of a ureylene group (N,N-dehydrourea, a dehydrogenated derivative of urea). The side chains R1 and R2 distinguish various sulfonylureas. Sulfonylureas as the most widely used herbicide.

Exenatide, sold under the brand name Byetta and Bydureon among others, is a medication used to treat diabetes mellitus type 2. It is used together with diet, exercise, and potentially other antidiabetic medication. It is a treatment option after metformin and sulfonylureas. It is given by injection under the skin twice daily or once weekly.

Inhibitors of dipeptidyl peptidase 4 are a class of oral hypoglycemics that block the enzyme dipeptidyl peptidase-4 (DPP-4). They can be used to treat diabetes mellitus type 2.

Sitagliptin, sold under the brand name Januvia among others, is an anti-diabetic medication used to treat type 2 diabetes. In the United Kingdom it is listed as less preferred than metformin or a sulfonylurea. It is taken by mouth. It is also available in the fixed-dose combination medication sitagliptin/metformin.

Saxagliptin, sold under the brand name Onglyza, is an oral hypoglycemic of the dipeptidyl peptidase-4 (DPP-4) inhibitor class. Early development was solely by Bristol-Myers Squibb; in 2007 AstraZeneca joined with Bristol-Myers Squibb to co-develop the final compound and collaborate on the marketing of the drug.
Teplizumab, sold under the brand name Tzield, is a humanized anti-CD3 monoclonal antibody that is the first approved treatment indicated to delay the onset of stage 3 type 1 diabetes (T1D) in people with stage 2 T1D.
Otelixizumab, also known as TRX4, is a monoclonal antibody, which is being developed for the treatment of type 1 diabetes and other autoimmune diseases. The antibody is being developed by Tolerx, Inc. in collaboration with GlaxoSmithKline and is being manufactured by Abbott Laboratories.
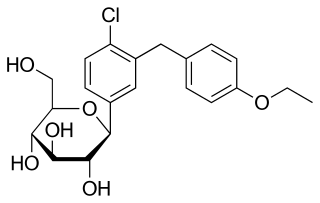
Dapagliflozin, sold under the brand names Farxiga (US) and Forxiga (EU) among others, is a medication used to treat type 2 diabetes. It is also used to treat adults with heart failure and chronic kidney disease.

Lorcaserin, marketed under the brand name Belviq, was a weight-loss drug developed by Arena Pharmaceuticals. It reduces appetite by activating a type of serotonin receptor known as the 5-HT2C receptor in a region of the brain called the hypothalamus, which is known to control appetite. It was approved in 2012, and in 2020, it was removed from the market in the United States due to an increased risk of cancer detected in users of Belviq.

Saroglitazar is a drug for the treatment of type 2 diabetes mellitus and dyslipidemia. It is approved for use in India by the Drug Controller General of India. Saroglitazar is indicated for the treatment of diabetic dyslipidemia and hypertriglyceridemia with type 2 diabetes mellitus not controlled by statin therapy. In clinical studies, saroglitazar has demonstrated reduction of triglycerides (TG), LDL cholesterol, VLDL cholesterol, non-HDL cholesterol and an increase in HDL cholesterol a characteristic hallmark of atherogenic diabetic dyslipidemia (ADD). It has also shown anti-diabetic medication properties by reducing the fasting plasma glucose and HBA1c in diabetes patients.

Diabetes mellitus, often known simply as diabetes, is a group of common endocrine diseases characterized by sustained high blood sugar levels. Diabetes is due to either the pancreas not producing enough insulin, or the cells of the body becoming unresponsive to the hormone's effects. Classic symptoms include thirst, polyuria, weight loss, and blurred vision. If left untreated, the disease can lead to various health complications, including disorders of the cardiovascular system, eye, kidney, and nerves. Untreated or poorly treated diabetes accounts for approximately 1.5 million deaths every year.

Dulaglutide, sold under the brand name Trulicity among others, is a medication used for the treatment of type 2 diabetes in combination with diet and exercise. It is also approved in the United States for the reduction of major adverse cardiovascular events in adults with type 2 diabetes who have established cardiovascular disease or multiple cardiovascular risk factors. It is a once-weekly injection.
SGLT2 inhibitors, also called gliflozins or flozins, are a class of medications that inhibit sodium-glucose transport proteins in the nephron, unlike SGLT1 inhibitors that perform a similar function in the intestinal mucosa. The foremost metabolic effect of this is to inhibit reabsorption of glucose in the kidney and therefore lower blood sugar. They act by inhibiting sodium-glucose transport protein 2 (SGLT2). SGLT2 inhibitors are used in the treatment of type 2 diabetes. Apart from blood sugar control, gliflozins have been shown to provide significant cardiovascular benefit in people with type 2 diabetes. As of 2014, several medications of this class had been approved or were under development. In studies on canagliflozin, a member of this class, the medication was found to enhance blood sugar control as well as reduce body weight and systolic and diastolic blood pressure.

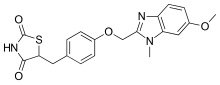
Trelagliptin is a pharmaceutical drug used for the treatment of type 2 diabetes.

Gosogliptin is a drug for the treatment of type II diabetes. It is in the class of dipeptidyl peptidase-4 (DPP-4) inhibitors. It was discovered and developed through Phase 1 and Phase 2 by Pfizer. The crystal structure of DPP-4 in complex with gosogliptin is available. Its metabolism, excretion and pharmacokinetics in rat, dog and human have been described. A cost efficient route has been published. Other studies including Phase 3 studies were conducted in Russia. It is approved for use in Russia.

Tirzepatide, sold under the brand name Mounjaro among others, is an antidiabetic medication used for the treatment of type 2 diabetes and for weight loss. Tirzepatide is administered through subcutaneous injection.