 | |
| Clinical data | |
|---|---|
| Pronunciation | /tɛlmɪˈsɑːrtən/ |
| Trade names | Micardis, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a601249 |
| License data |
|
| Pregnancy category | |
| Routes of administration | By mouth |
| Drug class | Angiotensin II receptor antagonist |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 42–100% |
| Protein binding | >99.5% |
| Metabolism | Minimal liver (glucuronidation) |
| Elimination half-life | 24 hours |
| Excretion | Feces 97% |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.149.347 |
| Chemical and physical data | |
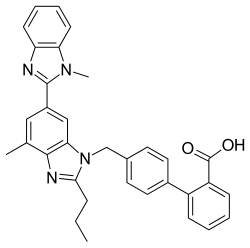
| Formula | C33H30N4O2 |
| Molar mass | 514.629 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Telmisartan, sold under the brand name Micardis among others, is a medication used to treat high blood pressure and heart failure. [2] [5] It is a reasonable initial treatment for high blood pressure. [5] It is taken by mouth. [5]
Contents
- Medical uses
- Contraindications
- Side effects
- Interactions
- Pharmacology
- Mechanism of action
- Pharmacokinetics
- History
- Society and culture
- Research
- References
- Further reading
Common side effects include upper respiratory tract infections, diarrhea, and back pain. [5] Serious side effects may include kidney problems, low blood pressure, and angioedema. [5] Use in pregnancy may harm the baby and use when breastfeeding is not recommended. [1] It is an angiotensin II receptor blocker and works by blocking the effects of angiotensin II. [5]
Telmisartan was patented in 1991 and came into medical use in 1999. [6] It is available as a generic medication. [7] In 2023, it was the 184th most commonly prescribed medication in the United States, with more than 2 million prescriptions. [8] [9] It is available in combination with hydrochlorothiazide as telmisartan/hydrochlorothiazide; [10] with cilnidipine as telmisartan/cilnidipine; [11] and with amlodipine as telmisartan/amlodipine. [5] [12]