A protease is an enzyme that catalyzes proteolysis, breaking down proteins into smaller polypeptides or single amino acids, and spurring the formation of new protein products. They do this by cleaving the peptide bonds within proteins by hydrolysis, a reaction where water breaks bonds. Proteases are involved in many biological functions, including digestion of ingested proteins, protein catabolism, and cell signaling.
Pepsin is an endopeptidase that breaks down proteins into smaller peptides. It is produced in the gastric chief cells of the stomach lining and is one of the main digestive enzymes in the digestive systems of humans and many other animals, where it helps digest the proteins in food. Pepsin is an aspartic protease, using a catalytic aspartate in its active site.

An osteoclast is a type of bone cell that breaks down bone tissue. This function is critical in the maintenance, repair, and remodeling of bones of the vertebral skeleton. The osteoclast disassembles and digests the composite of hydrated protein and mineral at a molecular level by secreting acid and a collagenase, a process known as bone resorption. This process also helps regulate the level of blood calcium.

MyoD, also known as myoblast determination protein 1, is a protein in animals that plays a major role in regulating muscle differentiation. MyoD, which was discovered in the laboratory of Harold M. Weintraub, belongs to a family of proteins known as myogenic regulatory factors (MRFs). These bHLH transcription factors act sequentially in myogenic differentiation. Vertebrate MRF family members include MyoD1, Myf5, myogenin, and MRF4 (Myf6). In non-vertebrate animals, a single MyoD protein is typically found.
A mitogen-activated protein kinase is a type of protein kinase that is specific to the amino acids serine and threonine. MAPKs are involved in directing cellular responses to a diverse array of stimuli, such as mitogens, osmotic stress, heat shock and proinflammatory cytokines. They regulate cell functions including proliferation, gene expression, differentiation, mitosis, cell survival, and apoptosis.
Granzyme B (GrB) is a serine protease most commonly found in the granules of natural killer cells and cytotoxic T cells. It is secreted by these cells along with the pore forming protein perforin to mediate apoptosis in target cells.

Cathepsins are proteases found in all animals as well as other organisms. There are approximately a dozen members of this family, which are distinguished by their structure, catalytic mechanism, and which proteins they cleave. Most of the members become activated at the low pH found in lysosomes. Thus, the activity of this family lies almost entirely within those organelles. There are, however, exceptions such as cathepsin K, which works extracellularly after secretion by osteoclasts in bone resorption. Cathepsins have a vital role in mammalian cellular turnover.

A calpain is a protein belonging to the family of calcium-dependent, non-lysosomal cysteine proteases expressed ubiquitously in mammals and many other organisms. Calpains constitute the C2 family of protease clan CA in the MEROPS database. The calpain proteolytic system includes the calpain proteases, the small regulatory subunit CAPNS1, also known as CAPN4, and the endogenous calpain-specific inhibitor, calpastatin.
Biological crosstalk refers to instances in which one or more components of one signal transduction pathway affects another. This can be achieved through a number of ways with the most common form being crosstalk between proteins of signaling cascades. In these signal transduction pathways, there are often shared components that can interact with either pathway. A more complex instance of crosstalk can be observed with transmembrane crosstalk between the extracellular matrix (ECM) and the cytoskeleton.

The bafilomycins are a family of macrolide antibiotics produced from a variety of Streptomycetes. Their chemical structure is defined by a 16-membered lactone ring scaffold. Bafilomycins exhibit a wide range of biological activity, including anti-tumor, anti-parasitic, immunosuppressant and anti-fungal activity. The most used bafilomycin is bafilomycin A1, a potent inhibitor of cellular autophagy. Bafilomycins have also been found to act as ionophores, transporting potassium K+ across biological membranes and leading to mitochondrial damage and cell death.
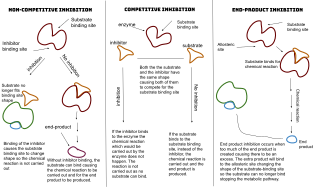
An enzyme inhibitor is a molecule that binds to an enzyme and decreases its activity. By binding to enzymes' active sites, inhibitors reduce the compatibility of substrate and enzyme and this leads to the inhibition of Enzyme-Substrate complexes' formation, preventing the catalysis of reactions and decreasing the amount of product produced by a reaction. It can be said that as the concentration of enzyme inhibitors increases, the rate of enzyme activity decreases, and thus, the amount of product produced is inversely proportional to the concentration of inhibitor molecules. Since blocking an enzyme's activity can kill a pathogen or correct a metabolic imbalance, many drugs are enzyme inhibitors. They are also used in pesticides. Not all molecules that bind to enzymes are inhibitors; enzyme activators bind to enzymes and increase their enzymatic activity, while enzyme substrates bind and are converted to products in the normal catalytic cycle of the enzyme.

Cathepsin S is a protein that in humans is encoded by the CTSS gene. Transcript variants utilizing alternative polyadenylation signals exist for this gene.

Aspartic proteases are a catalytic type of protease enzymes that use an activated water molecule bound to one or more aspartate residues for catalysis of their peptide substrates. In general, they have two highly conserved aspartates in the active site and are optimally active at acidic pH. Nearly all known aspartyl proteases are inhibited by pepstatin.

Cathepsin B belongs to a family of lysosomal cysteine proteases and plays an important role in intracellular proteolysis. In humans, cathepsin B is encoded by the CTSB gene. Cathepsin B is upregulated in certain cancers, in pre-malignant lesions, and in various other pathological conditions.

ETS Like-1 protein Elk-1 is a protein that in humans is encoded by the ELK1. Elk-1 functions as a transcription activator. It is classified as a ternary complex factor (TCF), a subclass of the ETS family, which is characterized by a common protein domain that regulates DNA binding to target sequences. Elk1 plays important roles in various contexts, including long-term memory formation, drug addiction, Alzheimer's disease, Down syndrome, breast cancer, and depression.

Cathepsin D is a protein that in humans is encoded by the CTSD gene. This gene encodes a lysosomal aspartyl protease composed of a protein dimer of disulfide-linked heavy and light chains, both produced from a single protein precursor. Cathepsin D is an aspartic endo-protease that is ubiquitously distributed in lysosomes. The main function of cathepsin D is to degrade proteins and activate precursors of bioactive proteins in pre-lysosomal compartments. This proteinase, which is a member of the peptidase A1 family, has a specificity similar to but narrower than that of pepsin A. Transcription of the CTSD gene is initiated from several sites, including one that is a start site for an estrogen-regulated transcript. Mutations in this gene are involved in the pathogenesis of several diseases, including breast cancer and possibly Alzheimer disease. Homozygous deletion of the CTSD gene leads to early lethality in the postnatal phase. Deficiency of CTSD gene has been reported an underlying cause of neuronal ceroid lipofuscinosis (NCL).

Renin inhibitors are pharmaceutical drugs inhibiting the activity of renin that is responsible for hydrolyzing angiotensinogen to angiotensin I, which in turn reduces the formation of angiotensin II that facilitates blood pressure.

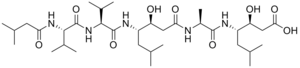
Statine is a gamma amino acid that occurs twice in the sequence of pepstatin, a protease inhibitor that is active against pepsin and other acid proteases. It is thought to be responsible for the inhibitory activity of pepstatin because it mimics the tetrahedral transition state of peptide catalysis.

Glutamic proteases are a group of proteolytic enzymes containing a glutamic acid residue within the active site. This type of protease was first described in 2004 and became the sixth catalytic type of protease. Members of this group of protease had been previously assumed to be an aspartate protease, but structural determination showed it to belong to a novel protease family. The first structure of this group of protease was scytalidoglutamic peptidase, the active site of which contains a catalytic dyad, glutamic acid (E) and glutamine (Q), which give rise to the name eqolisin. This group of proteases are found primarily in pathogenic fungi affecting plant and human.

Cytotrienin A is a secondary metabolite isolated from Streptomyces sp. RK95-74 isolated from soil in Japan in 1997. Cyt A is an ansamycin. Cytotrienin A induces apoptosis on HL-60 cells, as well as inhibiting translation in eukaryotes by inhibiting eukaryotic elongation factor 1A (eEF1A), which can act as an oncogene. These functions lead to the potential of the microbial metabolite acting as an anticancer agent, specifically for blood cancers, as it has proved to be more effective with leukemic cell lines. Cyt A is thought to induce apoptosis by activating c-Jun N-terminal Kinase (JNK), p38 mitogen-activated protein kinase (MAPK), and p36 myelin basic protein (MBP) kinase.