Angiotensin-converting-enzyme inhibitors are a class of medication used primarily for the treatment of high blood pressure and heart failure. They work by causing relaxation of blood vessels as well as a decrease in blood volume, which leads to lower blood pressure and decreased oxygen demand from the heart.

Atrial natriuretic peptide (ANP) or atrial natriuretic factor (ANF) is a natriuretic peptide hormone secreted from the cardiac atria that in humans is encoded by the NPPA gene. Natriuretic peptides are a family of hormone/paracrine factors that are structurally related. The main function of ANP is causing a reduction in expanded extracellular fluid (ECF) volume by increasing renal sodium excretion. ANP is synthesized and secreted by cardiac muscle cells in the walls of the atria in the heart. These cells contain volume receptors which respond to increased stretching of the atrial wall due to increased atrial blood volume.

Bradykinin (BK) is a peptide that promotes inflammation. It causes arterioles to dilate (enlarge) via the release of prostacyclin, nitric oxide, and endothelium-derived hyperpolarizing factor and makes veins constrict, via prostaglandin F2, thereby leading to leakage into capillary beds, due to the increased pressure in the capillaries. Bradykinin is a physiologically and pharmacologically active peptide of the kinin group of proteins, consisting of nine amino acids.
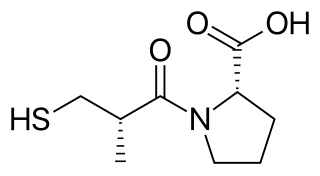
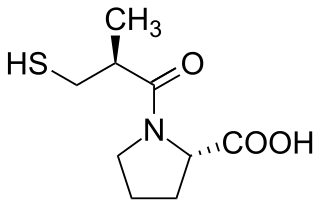
Captopril, sold under the brand name Capoten among others, is an angiotensin-converting enzyme (ACE) inhibitor used for the treatment of hypertension and some types of congestive heart failure. Captopril was the first oral ACE inhibitor found for the treatment of hypertension. It does not cause fatigue as associated with beta-blockers. Due to the adverse drug event of causing hyperkalemia, as seen with most ACE Inhibitors, the medication is usually paired with a diuretic.

Angioedema is an area of swelling (edema) of the lower layer of skin and tissue just under the skin or mucous membranes. The swelling may occur in the face, tongue, larynx, abdomen, or arms and legs. Often it is associated with hives, which are swelling within the upper skin. Onset is typically over minutes to hours.
The kinin–kallikrein system or simply kinin system is a poorly understood hormonal system with limited available research. It consists of blood proteins that play a role in inflammation, blood pressure control, coagulation and pain. Its important mediators bradykinin and kallidin are vasodilators and act on many cell types. Clinical symptoms include marked weakness, tachycardia, fever, leukocytosis and acceleration of ESR.

Neprilysin, also known as membrane metallo-endopeptidase (MME), neutral endopeptidase (NEP), cluster of differentiation 10 (CD10), and common acute lymphoblastic leukemia antigen (CALLA) is an enzyme that in humans is encoded by the MME gene. Neprilysin is a zinc-dependent metalloprotease that cleaves peptides at the amino side of hydrophobic residues and inactivates several peptide hormones including glucagon, enkephalins, substance P, neurotensin, oxytocin, and bradykinin. It also degrades the amyloid beta peptide whose abnormal folding and aggregation in neural tissue has been implicated as a cause of Alzheimer's disease. Synthesized as a membrane-bound protein, the neprilysin ectodomain is released into the extracellular domain after it has been transported from the Golgi apparatus to the cell surface.

Urodilatin (URO) is a hormone that causes natriuresis by increasing renal blood flow. It is secreted in response to increased mean arterial pressure and increased blood volume from the cells of the distal tubule and collecting duct. It is important in oliguric patients as it lowers serum creatinine and increases urine output.
Drug-induced angioedema is a known complication of the use of angiotensin-converting enzyme (ACE) inhibitors, angiotensin II antagonists (ARBs), and Angiotensin-Neprilysin Inhibitor LCZ969. The angioedema appears to be dose dependent as it may resolve with decreased dose.
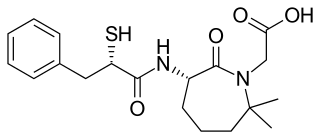
Gemopatrilat (INN) is an experimental drug that was never marketed. It acts as a vasopeptidase inhibitor. It inhibits both angiotensin-converting enzyme (ACE) and neutral endopeptidase (neprilysin).

Ecadotril is a neutral endopeptidase inhibitor and determined by the presence of peptidase family M13 as a neutral endopeptidase inhibited by phosphoramidon. Ecadotril is the (S)-enantiomer of racecadotril. NEP-like enzymes include the endothelin-converting enzymes. The peptidase M13 family believed to activate or inactivate oligopeptide (pro)-hormones such as opioid peptides, neprilysin is another member of this group, in the case of the metallopeptidases and aspartic, the nucleophiles clan or family for example MA, is an activated water molecule. The peptidase domain for members of this family also contains a bacterial member and resembles that of thermolysin the predicted active site residues for members of this family and thermolysin occur in the motif HEXXH. Thermolysin complexed with the inhibitor (S)-thiorphan are isomeric thiol-containing inhibitors of endopeptidase EC 24-11.

Candoxatril is the orally active prodrug of candoxatrilat (UK-73967).

An Oligopeptidase is an enzyme that cleaves peptides but not proteins. This property is due to its structure: the active site of this enzyme is located at the end of a narrow cavity which can only be reached by peptides.

Kelatorphan is a drug which acts as a powerful and complete inhibitor of nearly all of the enzymes responsible for catabolism of the endogenous enkephalins, including neutral endopeptidase (NEP), dipeptidyl peptidase III (DPP3), aminopeptidase N (APN), and angiotensin-converting enzyme (ACE). In mice, with the intracerebroventricular co-administration of a 50 µg dose of kelatorphan (this route is necessary because kelatorphan is incapable of crossing the blood-brain-barrier) hence alongside exogenous [Met]enkephalin (ED50 approximately 10 ng), it potentiated the analgesic effects of the latter by 50,000 times. Kelatorphan also displays potent antinociceptive effects alone, and does not depress respiration, although at high doses it actually increases it.
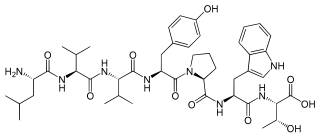
Spinorphin is an endogenous, non-classical opioid peptide of the hemorphin family first isolated from the bovine spinal cord (hence the prefix spin-) and acts as a regulator of the enkephalinases, a class of enzymes that break down endogenous the enkephalin peptides. It does so by inhibiting the enzymes aminopeptidase N (APN), dipeptidyl peptidase III (DPP3), angiotensin-converting enzyme (ACE), and neutral endopeptidase (NEP). Spinorphin is a heptapeptide and has the amino acid sequence Leu-Val-Val-Tyr-Pro-Trp-Thr (LVVYPWT). It has been observed to possess antinociceptive, antiallodynic, and anti-inflammatory properties. The mechanism of action of spinorphin has not been fully elucidated (i.e., how it acts to inhibit the enkephalinases), but it has been found to act as an antagonist of the P2X3 receptor, and as a weak partial agonist/antagonist of the FP1 receptor.

Tynorphin is a synthetic opioid peptide which is a potent and competitive inhibitor of the enkephalinase class of enzymes which break down the endogenous enkephalin peptides. It specifically inactivates dipeptidyl aminopeptidase III (DPP3) with very high efficacy, but also inhibits neutral endopeptidase (NEP), aminopeptidase N (APN), and angiotensin-converting enzyme (ACE) to a lesser extent. It has a pentapeptide structure with the amino acid sequence Val-Val-Tyr-Pro-Trp (VVYPW).
Sacubitril/valsartan, sold under the brand name Entresto, is a fixed-dose combination medication for use in heart failure. It consists of the neprilysin inhibitor sacubitril and the angiotensin receptor blocker valsartan. The combination is sometimes described as an "angiotensin receptor-neprilysin inhibitor" (ARNi). It is recommended for use as a replacement for an ACE inhibitor or an angiotensin receptor blocker in people with heart failure with reduced ejection fraction.

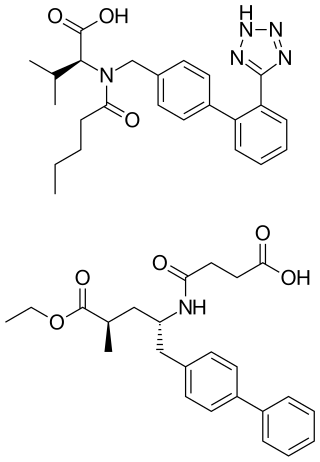
Sacubitril is an antihypertensive drug used in combination with valsartan. The combination drug sacubitril/valsartan, known during trials as LCZ696 and marketed under the brand name Entresto, is a treatment for heart failure. It was approved under the FDA's priority review process for use in heart failure on July 7, 2015.
An endopeptidase inhibitor is a drug that inhibits one or more endopeptidase enzymes. Endopeptidases are one of two types of proteases, the other being exopeptidases. Endopeptidases cleave peptide bonds of non-terminal amino acids, whereas exopeptidases break terminal bonds, resulting in the release of a single amino acid or dipeptide from the peptide chain.

Angiotensin (1-7) is an active heptapeptide of the renin–angiotensin system (RAS).

















