 | |
| Clinical data | |
|---|---|
| Trade names | Univasc |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a695018 |
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 13-22% |
| Protein binding | 90% |
| Metabolism | Hepatic (active metabolite, moexiprilat) |
| Elimination half-life | 1 hour; 2-9 hours (active metabolite) |
| Excretion | 50% (faeces), 13% (urine) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
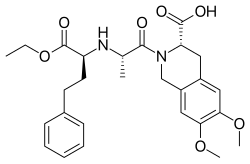
| Formula | C27H34N2O7 |
| Molar mass | 498.576 g·mol−1 |
| | |
Moexipril was an angiotensin converting enzyme inhibitor (ACE inhibitor) [1] used for the treatment of hypertension and congestive heart failure. Moexipril can be administered alone or with other antihypertensives or diuretics. [2]
Contents
It works by inhibiting the conversion of angiotensin I to angiotensin II. [3]
It was patented in 1980 and approved for medical use in 1995. [4] Moexipril is available from Schwarz Pharma under the trade name Univasc. [3] [5]
