In biochemistry, a metabolite is an intermediate or end product of metabolism. The term is usually used for small molecules. Metabolites have various functions, including fuel, structure, signaling, stimulatory and inhibitory effects on enzymes, catalytic activity of their own, defense, and interactions with other organisms.

Quinapril, sold under the brand name Accupril by the Pfizer corporation. It a medication used to treat high blood pressure (hypertension), heart failure, and diabetic kidney disease. It is a first line treatment for high blood pressure. It is taken by mouth.

Nordazepam is a 1,4-benzodiazepine derivative. Like other benzodiazepine derivatives, it has amnesic, anticonvulsant, anxiolytic, muscle relaxant, and sedative properties. However, it is used primarily in the treatment of anxiety disorders. It is an active metabolite of diazepam, chlordiazepoxide, clorazepate, prazepam, pinazepam, and medazepam.

Adinazolam is a tranquilizer of the triazolobenzodiazepine (TBZD) class, which are benzodiazepines (BZDs) fused with a triazole ring. It possesses anxiolytic, anticonvulsant, sedative, and antidepressant properties. Adinazolam was developed by Jackson B. Hester, who was seeking to enhance the antidepressant properties of alprazolam, which he also developed. Adinazolam was never FDA approved and never made available to the public market; however, it has been sold as a designer drug.

Ethyl loflazepate is a drug which is a benzodiazepine derivative. It possesses anxiolytic, anticonvulsant, sedative and skeletal muscle relaxant properties. In animal studies it was found to have low toxicity, although in rats evidence of pulmonary phospholipidosis occurred with pulmonary foam cells developing with long-term use of very high doses. Its elimination half-life is 51–103 hours. Its mechanism of action is similar to other benzodiazepines. Ethyl loflazepate also produces an active metabolite which is stronger than the parent compound. Ethyl loflazepate was designed to be a prodrug for descarboxyloflazepate, its active metabolite. It is the active metabolite which is responsible for most of the pharmacological effects rather than ethyl loflazepate. The main metabolites of ethyl loflazepate are descarbethoxyloflazepate, loflazepate and 3-hydroxydescarbethoxyloflazepate. Accumulation of the active metabolites of ethyl loflazepate are not affected by those with kidney failure or impairment. The symptoms of an overdose of ethyl loflazepate include sleepiness, agitation and ataxia. Hypotonia may also occur in severe cases. These symptoms occur much more frequently and severely in children. Death from therapeutic maintenance doses of ethyl loflazepate taken for 2 – 3 weeks has been reported in 3 elderly patients. The cause of death was asphyxia due to benzodiazepine toxicity. High doses of the antidepressant fluvoxamine may potentiate the adverse effects of ethyl loflazepate.

Desmetramadol (INN), also known as O-desmethyltramadol (O-DSMT), is an opioid analgesic and the main active metabolite of tramadol. Tramadol is demethylated by the liver enzyme CYP2D6 to desmetramadol in the same way as codeine, and so similarly to the variation in effects seen with codeine, individuals who have a less active form of CYP2D6 will tend to have reduced analgesic effects from tramadol. Because desmetramadol itself does not need to be metabolized to induce an analgesic effect, it can be used in individuals with low CYP2D6 activity unlike tramadol.
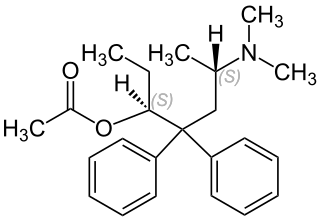
Levacetylmethadol (INN), levomethadyl acetate (USAN), OrLAAM or levo-α-acetylmethadol (LAAM) is a synthetic opioid similar in structure to methadone. It has a long duration of action due to its active metabolites.

Flutazolam is a drug which is a benzodiazepine derivative. It was invented in Japan, and this is the main country in which it has been used medically. It has sedative, muscle relaxant, anticonvulsant, and anxiolytic effects similar to those produced by other benzodiazepine derivatives, and though it is around the same potency as diazepam, it produces a more marked sedation and impaired coordination. It is indicated for the treatment of insomnia. Its major active metabolite is n-desalkylflurazepam, also known as norflurazepam, which is also a principal metabolite of flurazepam. While flutazolam has a very short half-life of only 3.5 hours, n-desalkylflurazepam has a long half-life of between 47–100 hours.

Norcocaine is a minor metabolite of cocaine. It is the only confirmed pharmacologically active metabolite of cocaine, although salicylmethylecgonine is also speculated to be an active metabolite. The local anesthetic potential of norcocaine has been shown to be higher than that of cocaine, however cocaine continues to be more widely used. Norcocaine used for research purposes is typically synthesized from cocaine. Several methods for the synthesis have been described.

Ceftiofur is an antibiotic of the cephalosporin type, licensed for use in veterinary medicine. It was first described in 1987. It is marketed by pharmaceutical company Zoetis as Excenel, Naxcel, and Excede and is also the active ingredient in that company's Spectramast LC and Spectramast DC product.
An active metabolite, or pharmacologically active metabolite is a biologically active metabolite of a xenobiotic substance, such as a drug or environmental chemical. Active metabolites may produce therapeutic effects, as well as harmful effects.
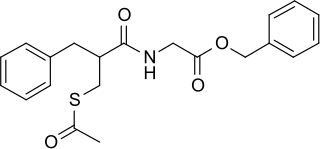
Racecadotril, also known as acetorphan, is an antidiarrheal medication which acts as a peripheral enkephalinase inhibitor. Unlike other opioid medications used to treat diarrhea, which reduce intestinal motility, racecadotril has an antisecretory effect — it reduces the secretion of water and electrolytes into the intestine. It is available in France and other European countries as well as most of South America and some South East Asian countries, but not in the United States. It is sold under the tradename Hidrasec, among others. Thiorphan is the active metabolite of racecadotril, which exerts the bulk of its inhibitory actions on enkephalinases.

Licarbazepine is a voltage-gated sodium channel blocker with anticonvulsant and mood-stabilizing effects that is related to oxcarbazepine. It is an active metabolite of oxcarbazepine. In addition, an enantiomer of licarbazepine, eslicarbazepine ((S)-(+)-licarbazepine), is an active metabolite of eslicarbazepine acetate. Oxcarbazepine and eslicarbazepine acetate are inactive on their own, and behave instead as prodrugs to licarbazepine and eslicarbazepine, respectively, to produce their therapeutic effects.

9-Nor-9β-hydroxyhexahydrocannabinol ,is a cannabinoid first discovered from early modifications to the structure of THC, in a search for the simplest compound that could still fulfill the binding requirements to produce cannabis-like activity.
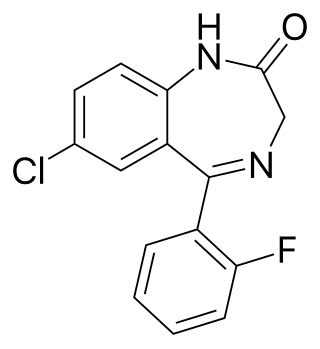
N-Desalkylflurazepam is a benzodiazepine analog and an active metabolite of several other benzodiazepine drugs including flurazepam, flutoprazepam, fludiazepam, midazolam, flutazolam, quazepam, and ethyl loflazepate. It is long-acting, prone to accumulation, and binds unselectively to the various benzodiazepine receptor subtypes. It has been sold as a designer drug from 2016 onward.

SL-75102, or progabide acid, is an active metabolite of progabide and an anticonvulsant GABA receptor agonist.

8,11-Dihydroxytetrahydrocannabinol (8β,11-diOH-Δ9-THC) is an active metabolite of THC, the main active component of cannabis. The 8β enantiomer retains psychoactive effects in animal studies with only slightly lower potency than THC, while the 8α enantiomer is much weaker. Both enantiomers have a shorter half-life in the body than 11-Hydroxy-THC, making 8,11-dihydroxy-THC potentially useful for drug testing to distinguish between recent cannabis use and use longer in the past.

11-Hydroxy-Delta-8-tetrahydrocannabinol is an active metabolite of Δ8-THC, a psychoactive cannabinoid found in small amounts in cannabis. It is an isomer of 11-OH-Δ9-THC, and is produced via the same metabolic pathway. It was the first cannabinoid metabolite discovered in 1970.

3'-Hydroxy-THC (3'-OH-Δ9-THC) is a minor active metabolite of THC, the main psychoactive component of cannabis. It is one of a number of metabolites of THC hydroxylated on the pentyl side chain, but while the other side-chain hydroxyl isomers are much weaker or inactive, the S enantiomer of 3'-OH-THC is several times more potent than THC itself, and while it is produced in smaller amounts than other active metabolites such as 11-Hydroxy-THC and 8,11-Dihydroxy-THC, it is thought to contribute to the overall pharmacological profile of cannabis.

11-Hydroxycannabinol (11-OH-CBN) is the main active metabolite of cannabinol (CBN), one of the active components of cannabis, and has also been isolated from cannabis itself. It is more potent than CBN itself, acting as an agonist of CB1 with around the same potency as THC, but is a weak antagonist at CB2.


















