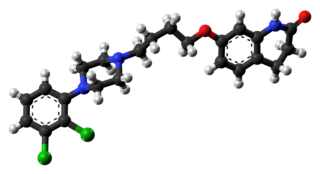
Aripiprazole, sold under the brand names Abilify and Aristada, among others, is an atypical antipsychotic. It is primarily used in the treatment of schizophrenia and bipolar disorder; other uses include as an add-on treatment in major depressive disorder and obsessive–compulsive disorder (OCD), tic disorders, and irritability associated with autism. Aripiprazole is taken by mouth or via injection into a muscle. A Cochrane review found low-quality evidence of effectiveness in treating schizophrenia.

Coprolalia is involuntary swearing or the involuntary utterance of obscene words or socially inappropriate and derogatory remarks. The word comes from the Greek κόπρος, meaning "dung, feces", and λαλιά "speech", from λαλεῖν "to talk".

A dopamine antagonist, also known as an anti-dopaminergic and a dopamine receptor antagonist (DRA), is a type of drug which blocks dopamine receptors by receptor antagonism. Most antipsychotics are dopamine antagonists, and as such they have found use in treating schizophrenia, bipolar disorder, and stimulant psychosis. Several other dopamine antagonists are antiemetics used in the treatment of nausea and vomiting.

Tetrabenazine is a drug for the symptomatic treatment of hyperkinetic movement disorders. It is sold under the brand names Nitoman and Xenazine among others. On August 15, 2008, the U.S. Food and Drug Administration approved the use of tetrabenazine to treat chorea associated with Huntington's disease. Although other drugs had been used "off label," tetrabenazine was the first approved treatment for Huntington's disease in the U.S. The compound has been known since the 1950s.

Dopaminergic means "related to dopamine", a common neurotransmitter. Dopaminergic substances or actions increase dopamine-related activity in the brain.

Amisulpride is an antiemetic and antipsychotic medication used at lower doses intravenously to prevent and treat postoperative nausea and vomiting; and at higher doses by mouth to treat schizophrenia and acute psychotic episodes. It is sold under the brand names Barhemsys and Solian, Socian, Deniban and others. At very low doses it is also used to treat dysthymia.

Agomelatine, sold under the brand names Valdoxan and Thymanax, among others, is an atypical antidepressant most commonly used to treat major depressive disorder and generalized anxiety disorder. One review found that it is as effective as other antidepressants with similar discontinuation rates overall but fewer discontinuations due to side effects. Another review also found it was similarly effective to many other antidepressants.

Dopamine receptor D1, also known as DRD1. It is one of the two types of D1-like receptor family — receptors D1 and D5. It is a protein that in humans is encoded by the DRD1 gene.

Istradefylline, sold under the brand name Nourianz, is a medication used as an add-on treatment to levodopa/carbidopa in adults with Parkinson's disease (PD) experiencing "off" episodes. Istradefylline reduces "off" periods resulting from long-term treatment with the antiparkinson drug levodopa. An "off" episode is a time when a patient's medications are not working well, causing an increase in PD symptoms, such as tremor and difficulty walking.
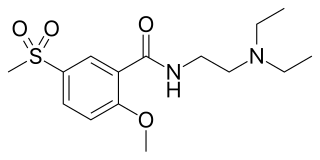
Tiapride is a drug that selectively blocks D2 and D3 dopamine receptors in the brain. It is used to treat a variety of neurological and psychiatric disorders including dyskinesia, alcohol withdrawal syndrome, negative symptoms of psychosis, and agitation and aggression in the elderly. A derivative of benzamide, tiapride is chemically and functionally similar to other benzamide antipsychotics such as sulpiride and amisulpride known for their dopamine antagonist effects.

Piquindone (Ro 22-1319) is an atypical antipsychotic with a tricyclic structure that was developed in the 1980s but was never marketed. It acts as a selective D2 receptor antagonist, though based on its effects profile its selectivity may be considered controversial. Unlike most other D2 receptor ligands, piquindone displays Na+-dependent binding, a property it shares with tropapride, zetidoline, and metoclopramide.
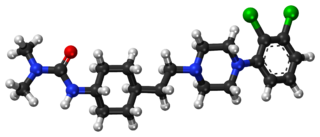
Cariprazine, sold under the brand name Vraylar among others, is an atypical antipsychotic developed by Gedeon Richter, which is used in the treatment of schizophrenia, bipolar mania, bipolar depression, and major depressive disorder. It acts primarily as a D3 and D2 receptor partial agonist, with a preference for the D3 receptor. Cariprazine is also a partial agonist at the serotonin 5-HT1A receptor and acts as an antagonist at 5-HT2B and 5-HT2A receptors, with high selectivity for the D3 receptor. It is taken by mouth.

Brexpiprazole, sold under the brand name Rexulti among others, is an atypical antipsychotic medication used for the treatment of major depressive disorder, schizophrenia, and agitation associated with dementia due to Alzheimer's disease.

Brilaroxazine, also known as oxaripiprazole, is an investigational atypical antipsychotic which is under development by Reviva Pharmaceuticals for the treatment of neuropsychiatric and inflammatory disorders. It has currently completed the first of two phase III clinical trials for schizophrenia. Reviva Pharmaceuticals also intends to investigate brilaroxazine for the treatment of bipolar disorder, major depressive disorder, attention deficit hyperactivity disorder (ADHD), irritability in autism, tics, psychosis/agitation associated with Alzheimer's disease, Parkinson's disease psychosis, as well as the inflammatory disorders pulmonary arterial hypertension (PAH), idiopathic pulmonary fibrosis (IPF), and psoriasis. The FDA granted brilaroxazine orphan drug designation for the treatment of PAH and IPF.
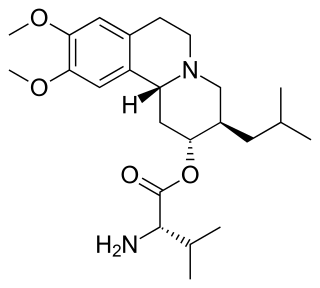
Valbenazine, sold under the brand name Ingrezza, is a medication used to treat tardive dyskinesia. It acts as a vesicular monoamine transporter 2 (VMAT2) inhibitor.

Dextromethorphan/bupropion (DXM/BUP), sold under the brand name Auvelity, is a combination medication for the treatment of major depressive disorder (MDD). Its active components are dextromethorphan (DXM) and bupropion. Patients who stayed on the medication had an average of 11% greater reduction in depressive symptoms than placebo in an FDA approval trial. It is taken as a tablet by mouth.
The dopamine hypothesis of stuttering attributes to the phenomenon of stuttering a hyperactive and disturbed dopaminergic signal transduction in the brain. The theory is derived from observations in medical neuroimaging and from the empirical response of some antipsychotics and their antagonistic effects on the dopamine receptor. However, it is important to outline that the hypothesis does not consider the excessive dopaminergic activity as the direct cause of stuttering; instead, this synaptic dysregulation is a symptom of a greater disorder that affects other brain pathways and structures.

Mevidalen (LY-3154207) is a dopaminergic drug which is under development for the treatment of Lewy body disease, including those with Parkinson's disease.

NNC 01-0687 (also known as ADX-10061, CEE-03-310, or NNC-687) is a selective dopamine D1-like receptor antagonist of the benzazepine group which was under development as an experimental antipsychotic for the treatment of schizophrenia but was never marketed. Its development for schizophrenia was discontinued due to lack of effectiveness in clinical trials.
Berupipam (INNTooltip International Nonproprietary Name; developmental code name NNC 22-0010) is a selective dopamine D1 receptor antagonist of the benzazepine group which was under development for the treatment of psychotic disorders but was never marketed. It reached phase 1 clinical trials prior to the discontinuation of its development.




















