A dopamine reuptake inhibitor (DRI) is a class of drug which acts as a reuptake inhibitor of the monoamine neurotransmitter dopamine by blocking the action of the dopamine transporter (DAT). Reuptake inhibition is achieved when extracellular dopamine not absorbed by the postsynaptic neuron is blocked from re-entering the presynaptic neuron. This results in increased extracellular concentrations of dopamine and increase in dopaminergic neurotransmission.
The vesicular monoamine transporter (VMAT) is a transport protein integrated into the membranes of synaptic vesicles of presynaptic neurons. It transports monoamine neurotransmitters – such as dopamine, serotonin, norepinephrine, epinephrine, and histamine – into the vesicles, which release the neurotransmitters into synapses, as chemical messages to postsynaptic neurons. VMATs utilize a proton gradient generated by V-ATPases in vesicle membranes to power monoamine import.

The norepinephrine transporter (NET), also known as noradrenaline transporter (NAT), is a protein that in humans is encoded by the solute carrier family 6 member 2 (SLC6A2) gene.
Neurotransmitter transporters are a class of membrane transport proteins that span the cellular membranes of neurons. Their primary function is to carry neurotransmitters across these membranes and to direct their further transport to specific intracellular locations. There are more than twenty types of neurotransmitter transporters.

Troparil is a stimulant drug used in scientific research. Troparil is a phenyltropane-based dopamine reuptake inhibitor (DRI) that is derived from methylecgonidine. Troparil is a few times more potent than cocaine as a dopamine reuptake inhibitor, but is less potent as a serotonin reuptake inhibitor, and has a duration spanning a few times longer, since the phenyl ring is directly connected to the tropane ring through a non-hydrolyzable carbon-carbon bond. The lack of an ester linkage removes the local anesthetic action from the drug, so troparil is a pure stimulant. This change in activity also makes troparil slightly less cardiotoxic than cocaine. The most commonly used form of troparil is the tartrate salt, but the hydrochloride and naphthalenedisulfonate salts are also available, as well as the free base.

Naphthylaminopropane, also known as naphthylisopropylamine (NIPA), is an experimental drug that was under investigation for the treatment of alcohol and stimulant addiction.
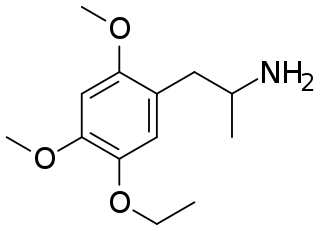
MME (2,4-dimethoxy-5-ethoxyamphetamine) is a lesser-known psychedelic drug. It is a dimethoxy-ethoxy analog of TMA-2. MME was first synthesized by Alexander Shulgin from ethylvanillin. In his book PiHKAL, the minimum dosage is listed as 40 mg and above, and the duration listed as 6–10 hours. Shulgin gives MME a ++ on the Shulgin Rating Scale.
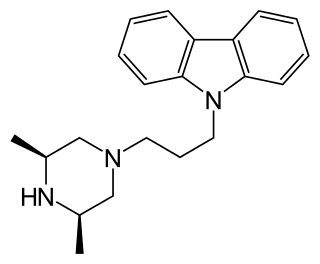
Rimcazole is an antagonist of the sigma receptor as well as a dopamine reuptake inhibitor. Sigma receptors are thought to be involved in the drug psychosis that can be induced by some drugs such as phencyclidine and cocaine, and rimcazole was originally researched as a potential antipsychotic with a different mechanism of action to traditional antipsychotic drugs. Trials proved inconclusive and rimcazole was not pursued for this application, but other sigma antagonists continue to be researched for a variety of potential applications. Rimcazole has been shown to reduce the effects of cocaine, and analogues of rimcazole have been shown to be highly effective at blocking the convulsions caused by cocaine overdose in animal models. Isothiocyanate derivatives of rimcazole acting as irreversible dopamine transporter (DAT) blockers have been developed.

RTI(-4229)-55, also called RTI-55 or iometopane, is a phenyltropane-based psychostimulant used in scientific research and in some medical applications. This drug was first cited in 1991. RTI-55 is a non-selective dopamine reuptake inhibitor derived from methylecgonidine. However, more selective analogs are derived by conversion to "pyrrolidinoamido" RTI-229, for instance. Due to the large bulbous nature of the weakly electron withdrawing iodo halogen atom, RTI-55 is the most strongly serotonergic of the simple para-substituted troparil based analogs. In rodents RTI-55 actually caused death at a dosage of 100 mg/kg, whereas RTI-51 and RTI-31 did not. Another notable observation is the strong propensity of RTI-55 to cause locomotor activity enhancements, although in an earlier study, RTI-51 was actually even stronger than RTI-55 in shifting baseline LMA. This observation serves to highlight the disparities that can arise between studies.

(–)-2β-Carboisopropoxy-3β-(4-iodophenyl)tropane is a stimulant drug used in scientific research, which was developed in the early 1990s. RTI-121 is a phenyltropane based, highly selective dopamine reuptake inhibitor and is derived from methylecgonidine. RTI-121 is a potent and long-lasting stimulant, producing stimulant effects for more than 10 hours after a single dose in mice which would limit its potential uses in humans, as it might have significant abuse potential if used outside a medical setting. However RTI-121 occupies the dopamine transporter more slowly than cocaine, and so might have lower abuse potential than cocaine itself.
In enzymology, a quinoline-4-carboxylate 2-oxidoreductase (EC 1.3.99.19) is an enzyme that catalyzes the chemical reaction
A neurotransmitter sodium symporter (NSS) (TC# 2.A.22) is type of neurotransmitter transporter that catalyzes the uptake of a variety of neurotransmitters, amino acids, osmolytes and related nitrogenous substances by a solute:Na+ symport mechanism. The NSS family is a member of the APC superfamily. Its constituents have been found in bacteria, archaea and eukaryotes.

The Gould–Jacobs reaction is an organic synthesis for the preparation of quinolines and 4‐hydroxyquinoline derivatives. The Gould–Jacobs reaction is a series of reactions. The series of reactions begins with the condensation/substitution of an aniline with alkoxy methylenemalonic ester or acyl malonic ester, producing anilidomethylenemalonic ester. Then through a 6 electron cyclization process, 4-hydroxy-3-carboalkoxyquinoline is formed, which exist mostly in the 4-oxo form. Saponification results in the formation of an acid. This step is followed by decarboxylation to give 4-hydroxyquinoline. The Gould–Jacobs reaction is effective for anilines with electron‐donating groups at the meta‐position.

Reuptake inhibitors (RIs) are a type of reuptake modulators. It is a drug that inhibits the plasmalemmal transporter-mediated reuptake of a neurotransmitter from the synapse into the pre-synaptic neuron. This leads to an increase in extracellular concentrations of the neurotransmitter and an increase in neurotransmission. Various drugs exert their psychological and physiological effects through reuptake inhibition, including many antidepressants and psychostimulants.
The molecular formula C14H17NO3 (molar mass : 247.28 g/mol) may refer to :

RTI-229, also known as (–)-3β-(4-iodophenyl)tropane-2β-pyrrolidine carboxamide and RTI-4229-229, is a potent and long-lasting stimulant drug which was developed in the 1990s as part of a large group of related analogues from the phenyltropane family. With the combination of two potent dopamine transporter (DAT) binding motifs attached to the tropane ring, the p-iodophenyl group at the 3β-position and a pyrrolidine carboxamide at 2β, RTI-229 has extremely high selectivity for the dopamine transporter and is one of the most DAT-selective compounds in the RTI series.

Metaphit is a research chemical that acts as an acylator of NMDARAn, sigma and DAT binding sites in the CNS. It is the m-isothiocyanate derivative of phencyclidine (PCP) and binds irreversibly to the PCP binding site on the NMDA receptor complex. However, later studies suggest the functionality of metaphit is mediated by sites not involved in PCP-induced passive avoidance deficit, and not related to the NMDA receptor complex. Metaphit was also shown to prevent d-amphetamine induced hyperactivity, while significantly depleting dopamine content in the nucleus accumbens. Metaphit was the first acylating ligand used to study the cocaine receptor. It is a structural isomer of the similar research compound fourphit, as it and metaphit both are isothiocyanate substituted derivatives of an analogous scaffold shared with PCP.
An excitatory amino acid reuptake inhibitor (EAARI) is a type of drug which inhibits the reuptake of the excitatory neurotransmitters glutamate and aspartate by blocking one or more of the excitatory amino acid transporters (EAATs).
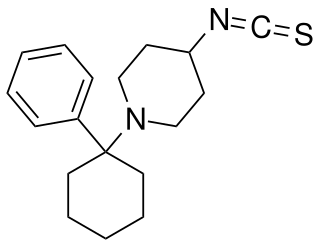
Fourphit, also known as 4-isothiocyanato-PCP, is an irreversible dopamine transporter (DAT) blocker and a reversible NMDA receptor antagonist. It blocks the binding of methylphenidate to the DAT in vitro, though apparently not in vivo. In any case, the drug reduces the stimulant-like effects of cocaine in animals, whilst producing mostly negligible behavioral effects itself. Fourphit is an acylating derivative of phencyclidine (PCP) and a positional isomer of metaphit (3-isothiocyanato-PCP).












