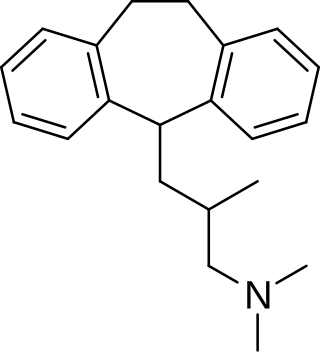
Butriptyline, sold under the brand name Evadyne among others, is a tricyclic antidepressant (TCA) that has been used in the United Kingdom and several other European countries for the treatment of depression but appears to no longer be marketed. Along with trimipramine, iprindole, and amoxapine, it has been described as an "atypical" or "second-generation" TCA due to its relatively late introduction and atypical pharmacology. It was very little-used compared to other TCAs, with the number of prescriptions dispensed only in the thousands.

Corbadrine, sold under the brand name Neo-Cobefrine and also known as levonordefrin and α-methylnorepinephrine, is a catecholamine sympathomimetic used as a topical nasal decongestant and vasoconstrictor in dentistry in the United States. It is usually used in a pre-mixed solution with local anesthetics, such as mepivacaine.

Medifoxamine, previously sold under the brand names Clédial and Gerdaxyl, is an atypical antidepressant with additional anxiolytic properties acting via dopaminergic and serotonergic mechanisms which was formerly marketed in France and Spain, as well as Morocco. The drug was first introduced in France sometime around 1990. It was withdrawn from the market in 1999 (Morocco) and 2000 (France) following incidences of hepatotoxicity.

Amidephrine, or amidefrine, sold under the brand name Fentrinol among others, is a selective α1-adrenergic receptor agonist which is described as an adrenergic or sympathomimetic, vasoconstrictor, and topical nasal decongestant used to treat allergic rhinitis. It is used as the mesylate salt, which has the generic names amidefrine mesilate and amidephrine mesylate. The drug is a substituted phenethylamine derivative and is also known as 3-methylsulfonamidyl-β-hydroxy-N-methylphenethylamine. As of 2000, it remained marketed only in Austria.

Nemonapride, also previously known as emonapride and sold under the brand name Emilace, is an atypical antipsychotic which is used in the treatment of schizophrenia. It is taken by mouth.
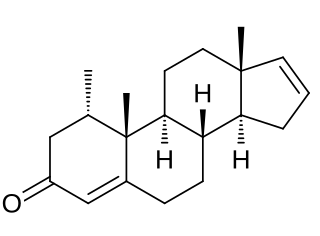
Delanterone, also known as 1α-methylandrosta-4,16-dien-3-one, is a steroidal antiandrogen described as an anti-acne agent which was never marketed. The compound showed poor efficacy as an antiandrogen in vivo in animals, suggestive of low activity or a short terminal half-life, and likely in relation to this was not further developed. It was described and characterized in the literature in 1977.

Oxendolone, sold under the brand names Prostetin and Roxenone, is an antiandrogen and progestin medication which is used in Japan in the treatment of enlarged prostate. However, this use is controversial due to concerns about its clinical efficacy. Oxendolone is not effective by mouth and must be given by injection into muscle.

Topterone, also known as 17α-propyltestosterone or as 17α-propylandrost-4-en-17β-ol-3-one, is a steroidal antiandrogen that was first reported in 1978 and was developed for topical administration but, due to poor effectiveness, was never marketed.
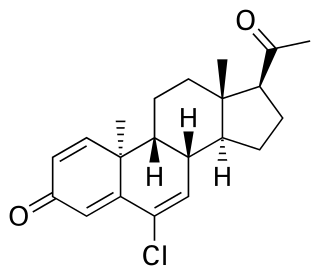
Trengestone, sold under the brand names Reteroid, Retroid, and Retrone, is a progestin medication which was formerly used to treat menstrual disorders but is now no longer marketed. It is taken by mouth.
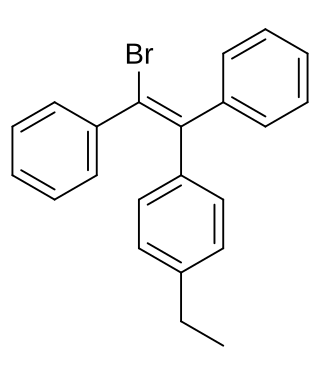
Broparestrol, also known as α-bromo-α,β-diphenyl-β-p-ethylphenylethylene (BDPE), is a synthetic, nonsteroidal selective estrogen receptor modulator (SERM) of the triphenylethylene group that has been used in Europe as a dermatological agent and for the treatment of breast cancer. The drug is described as slightly estrogenic and potently antiestrogenic, and inhibits mammary gland development and suppresses prolactin levels in animals. It is structurally related to clomifene and diethylstilbestrol. Broparestrol is a mixture of E- and Z- isomers, both of which are active, and are similarly antiestrogenic but, unlike broparestrol, were never marketed.
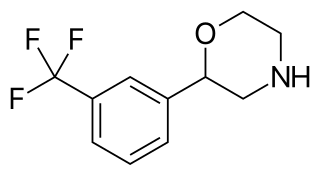
Flumexadol (INN) is a drug described and researched as a non-opioid analgesic which was never marketed. It has been found to act as an agonist of the serotonin 5-HT1A and 5-HT2C receptors and, to a much lesser extent, of the 5-HT2A receptor. According to Nilsson (2006) in a paper on 5-HT2C receptor agonists as potential anorectics, "The (+)-enantiomer of this compound showed [...] affinity for the 5-HT2C receptor (Ki) 25 nM) [...] and was 40-fold selective over the 5-HT2A receptor in receptor binding studies. The racemic version [...], also known as 1841 CERM, was originally reported to possess analgesic properties while no association with 5-HT2C receptor activity was mentioned." It is implied that flumexadol might be employable as an anorectic in addition to analgesic. Though flumexadol itself has never been approved for medical use, oxaflozane is a prodrug of the compound that was formerly used clinically in France as an antidepressant and anxiolytic agent.

Allenestrol, or allenoestrol, also known as α,α-dimethyl-β-ethylallenolic acid or as methallenestrilphenol, is a synthetic, nonsteroidal estrogen and a derivative of allenolic acid that was never marketed. A methyl ether of allenestrol, methallenestril (methallenestrol), is also an estrogen, but, in contrast to allenestrol, has been marketed.

Apparicine is a monoterpenoid tricyclic indole alkaloid. It is named after Apparicio Duarte, a Brazilian botanist who studied the Aspidosperma species from which apparicine was first isolated. It was the first member of the vallesamine group of indole alkaloids to be isolated and have its structure established, which was first published in 1965. It has also been known by the synonyms gomezine, pericalline, and tabernoschizine.

Estramustine is an estrogen and cytostatic antineoplastic agent which was never marketed. It is a carbamate derivative of estradiol and acts in part as a prodrug of estradiol in the body. Estramustine phosphate, the C17β phosphate ester of estramustine and a prodrug of estramustine, estromustine, estradiol, and estrone, is marketed and used in the treatment of prostate cancer.

Perlapine, sold under the brand names Hypnodine and Pipnodine, is a hypnotic and sedative of the tricyclic group which is marketed in Japan. It acts primarily as a potent antihistamine, and also has anticholinergic, antiserotonergic, antiadrenergic, and some antidopaminergic activity. The drug has relatively weak affinity for the dopamine D2 receptor (IC50Tooltip Half-maximal inhibitory concentration = 1,803 nM) and, in accordance, is said to be ineffective as an antipsychotic. However, it retains higher affinity for the dopamine D1 receptor (IC50 = 198 nM). Its IC50 values are 19 nM for the α1-adrenergic receptor, 4,945 nM for the α2-adrenergic receptor, and 70 nM for the serotonin 5-HT2A receptor. Perlapine is closely related to clotiapine, clozapine, fluperlapine, loxapine, and tilozepine.

Dopamantine is an antiparkinsonian drug of the adamantane group that developed for treatment of Parkinson's disease but was never marketed. It was developed and studied in the 1970s and was said to have reached early clinical trials.

Carmantadine is an antiparkinsonian agent of the adamantane group that was never marketed. It is structurally related to amantadine and shares some of its pharmacological actions. Another related drug is dopamantine. Carmantadine was first described by 1972 and is said to have reached early clinical trials.
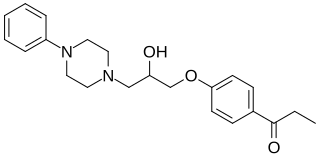
Centpropazine is an experimental antidepressant which was under development for the treatment major depressive disorder in India but was never marketed. It is described as having imipramine-like clinical effects, reversing reserpine-induced effects in animals, and potentiating amphetamine-induced effects in animals. The mechanism of action of centpropazine is unknown. The drug reached phase 3 clinical trials prior to the discontinuation of its development. It was first described in the scientific literature by 1980.

Tepirindole (INNTooltip International Nonproprietary Name; developmental code names RU-27592, HR-592) is a tryptamine-related atypical antipsychotic and major tranquilizer which was never marketed. It is similar in structure to tryptamines but is not technically a tryptamine itself and is instead a piperidinyl indole. The drug is said to act on dopamine D2, serotonin 5-HT2, and α1-adrenergic receptors. It is a potent dopamine receptor antagonist but reportedly has little propensity to cause catalepsy and has been said to potentially be useful in treating the negative symptoms of schizophrenia. The drug may also act as a potent serotonin receptor agonist. Tepirindole was first described in the literature by 1979.

Bufenadrine, also known as 2-tert-butyldiphenhydramine, is a drug described as an antiemetic, antihistamine, anticholinergic, and antiparkinsonian agent which was never marketed. It is the 2-tert-butyl analogue of diphenhydramine. The drug was found to produce stereoselective hepatotoxicity in animals and this led to the discontinuation of its development. Bufenadrine was first described in the literature by 1967. Its INNTooltip International Nonproprietary Name suffix "-drine" is generally for sympathomimetics but bufenadrine itself is not actually a sympathomimetic or related agent.




















