Fentanyl is a highly potent synthetic piperidine opioid primarily used as an analgesic. It is 20 to 40 times more potent than heroin and 100 times more potent than morphine; its primary clinical utility is in pain management for cancer patients and those recovering from painful surgeries. Fentanyl is also used as a sedative. Depending on the method of delivery, fentanyl can be very fast acting and ingesting a relatively small quantity can cause overdose. Fentanyl works by activating μ-opioid receptors. Fentanyl is sold under the brand names Actiq, Duragesic and Sublimaze, among others.
Carfentanil or carfentanyl, sold under the brand name Wildnil, is an extremely potent opioid analgesic used in veterinary medicine to anesthetize large animals such as elephants and rhinoceroses. It is typically administered in this context by tranquilizer dart. Carfentanil has also been used in humans to image opioid receptors. It has additionally been used as a recreational drug, typically by injection, insufflation, or inhalation. Deaths have been reported in association with carfentanil.
Kolokol-1 is a synthetic opioid developed for use as an aerosolizable incapacitating agent. The exact chemical structure has not yet been revealed by the Russian government. It was originally thought by some sources to be a derivative of the potent opioid fentanyl, most probably 3-methylfentanyl dissolved in an inhalational anaesthetic as an organic solvent. However, independent analysis of residues on the Moscow theater hostage crisis hostages' clothing or in one hostage's urine found no fentanyl or 3-methylfentanyl. Two much more potent and shorter-acting agents, carfentanil and remifentanil, were found in the samples. They concluded that the agent used in the Moscow theater hostage crisis contained two fentanyl derivatives much stronger than fentanyl itself, sprayed in an aerosol mist.

Xylazine is a structural analog of clonidine and an α2-adrenergic receptor agonist, sold under many trade names worldwide, most notably the Bayer brand name Rompun, as well as Anased, Sedazine and Chanazine.
Butyrophenone is an organic compound with the formula C6H5C(O)C3H7. It is a colorless liquid.

Droperidol is an antidopaminergic drug used as an antiemetic and as an antipsychotic. Droperidol is also often used as a rapid sedative in intensive-care treatment, and where "agitation aggression or violent behavior" are present.

Trifluperidol is a typical antipsychotic of the butyrophenone chemical class. It has general properties similar to those of haloperidol, but is considerably more potent by weight, and causes relatively more severe side effects, especially tardive dyskinesia and other extrapyramidal effects. It is used in the treatment of psychoses including mania and schizophrenia. It was discovered at Janssen Pharmaceutica in 1959.
Bromperidol, sold under the brand names Bromidol and Impromen among others, is a typical antipsychotic of the butyrophenone group which is used in the treatment of schizophrenia. It was discovered at Janssen Pharmaceutica in 1966. An ester prodrug, bromperidol decanoate, is a long-acting form of bromperidol used as a depot injectable.

Lofentanil or lofentanyl is one of the most potent opioid analgesics known and is an analogue of fentanyl, which was developed in 1960. It is most similar to the highly potent opioid carfentanil (4-carbomethoxyfentanyl), only slightly more potent. Lofentanil can be described as 3-methylcarfentanil, or 3-methyl-4-carbomethoxyfentanyl. While 3-methylfentanyl is considerably more potent than fentanyl itself, lofentanil is only slightly stronger than carfentanil. This suggests that substitution at both the 3 and 4 positions of the piperidine ring introduces steric hindrance which prevents μ-opioid affinity from increasing much further. As with other 3-substituted fentanyl derivatives such as ohmefentanyl, the stereoisomerism of lofentanil is very important, with some stereoisomers being much more potent than others.

Carpipramine is an atypical antipsychotic used for the treatment of schizophrenia and anxiety in France and Japan. In addition to its neuroleptic and anxiolytic effects, carpipramine also has hypnotic properties. It is structurally related to both tricyclics like imipramine and butyrophenones like haloperidol.

Moperone (Luvatren, since discontinued) is a typical antipsychotic of the butyrophenone class which is marketed in Japan for the treatment of schizophrenia. It is an antagonist for the D2 (Ki 0.7–1.9 nM), D3 (Ki 0.1–1 nM), and 5-HT2A (Ki 52 nM) receptors. It also has a high binding affinity for the sigma receptors.

Buphedrone, also known as α-methylamino-butyrophenone (MABP), is a stimulant of the phenethylamine and cathinone chemical classes that was first synthesized in 1928.

Lenperone (Elanone-V) is a typical antipsychotic of the butyrophenone chemical class. It was first reported as an anti-emetic in 1974, and its use in treatment of acute schizophrenia was reported in 1975. Related early antipsychotic agents include declenperone and milenperone.

2CB-Ind is a conformationally-restricted derivative of the phenethylamine hallucinogen 2C-B, discovered in 1974 by Alexander Shulgin. It acts as a moderately potent and selective agonist for the 5-HT2A and 5-HT2C receptors, but unlike the corresponding benzocyclobutene derivative TCB-2 which is considerably more potent than the parent compound 2C-B, 2CB-Ind is several times weaker, with racemic 2CB-Ind having a Ki of 47nM at the human 5-HT2A receptor, only slightly more potent than the mescaline analogue (R)-jimscaline.
Fentanyl/fluanisone is a veterinary combination drug consisting of fentanyl and fluanisone for use in mice, rats, rabbits and guinea pigs.

Butyrfentanyl or butyrylfentanyl is a potent short-acting synthetic opioid analgesic drug. It is an analog of fentanyl with around one quarter of its potency. One of the first mentions of this drug can be found in document written by The College on Problem of Drug Dependence, where it is mentioned as N-butyramide fentanyl analog. This document also states that the article describing its clinical effects was published in 1987. It is an agonist for the μ-opioid receptors.

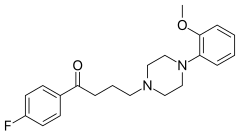
Acetylfentanyl is an opioid analgesic drug that is an analog of fentanyl. Studies have estimated acetylfentanyl to be 15 times more potent than morphine, which would mean that despite being somewhat weaker than fentanyl, it is nevertheless still several times stronger than pure heroin. It has never been licensed for medical use and instead has only been sold on the illicit drug market. Acetylfentanyl was discovered at the same time as fentanyl itself and had only rarely been encountered on the illicit market in the late 1980s. However, in 2013, Canadian police seized 3 kilograms of acetylfentanyl. As a μ-opioid receptor agonist, acetylfentanyl may serve as a direct substitute for oxycodone, heroin or other opioids. Common side effects of fentanyl analogs are similar to those of fentanyl itself, which include itching, nausea and potentially fatal respiratory depression. Fentanyl analogs have killed hundreds of people throughout Europe and the former Soviet republics since the most recent resurgence in use began in Estonia in the early 2000s, and novel derivatives continue to appear.
The word neuroleptic originates from the Greek word lepsis ("seizure"). Antipsychotics were investigated by the anesthesiologists De Castro and Mundeleer who coined the term neuroleptanalgesia, an anesthetic process that involves combining a major antipsychotic/neuroleptic or tranquilizer with a potent opioid analgesic to produce a detached, pain-free state. This technique was widely used from the 1960s onwards, initially using a combination of phenoperidine and haloperidol, which was subsequently replaced in the early 1980s by a combination of fentanyl and droperidol. Efforts were also made to develop compounds which combined both types of activity in a single molecule. Neuroleptanalgesia results in amnesia among some patients, but not all. The technique has become less popular with the advent of more modern procedural sedation drug combinations, though it is still rarely used today as a combination of 2.5 mg droperidol and 50 μg (micrograms) of fentanyl in a ratio of 50:1. This combination is characterized by immobility, analgesia, and variable amnesia.
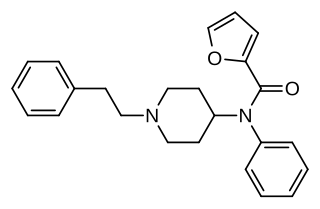
Furanylfentanyl (Fu-F) is an opioid analgesic that is an analog of fentanyl and has been sold as a designer drug. It has an ED50 value of 0.02 mg/kg in mice. This makes it approximately one fifth as potent as fentanyl.
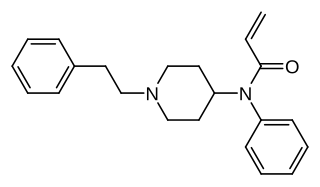
Acrylfentanyl (also known as acryloylfentanyl) is a highly potent opioid analgesic that is an analog of fentanyl and has been sold online as a designer drug. In animal studies the IC50 (the half maximal inhibitory concentration for acrylfentanyl to displace naloxone) is 1.4 nM, being slightly more potent than fentanyl itself (1.6 nM) as well as having a longer duration of action.