 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Fesobig , Toviaz |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a609021 |
| License data |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 52% (active metabolite) |
| Protein binding | 50% (active metabolite) |
| Metabolism | Liver (CYP2D6- and 3A4-mediated) |
| Elimination half-life | 7–8 hours (active metabolite) |
| Excretion | Kidney (70%) and fecal (7%) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.184.854 |
| Chemical and physical data | |
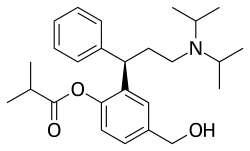
| Formula | C26H37NO3 |
| Molar mass | 411.586 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Fesoterodine (INN, used as the fumarate under the brand name Toviaz) is an antimuscarinic drug developed by Schwarz Pharma AG to treat overactive bladder syndrome (OAB). [2] It was approved by the European Medicines Agency in April 2007, [3] the US Food and Drug Administration on October 31, 2008 [4] and Health Canada on February 9, 2012. [5]
Fesoterodine is a prodrug. It is broken down into its active metabolite, desfesoterodine, by plasma esterases.