
Antiviral drugs are a class of medication used for treating viral infections. Most antivirals target specific viruses, while a broad-spectrum antiviral is effective against a wide range of viruses. Antiviral drugs are a class of antimicrobials, a larger group which also includes antibiotic, antifungal and antiparasitic drugs, or antiviral drugs based on monoclonal antibodies. Most antivirals are considered relatively harmless to the host, and therefore can be used to treat infections. They should be distinguished from virucides, which are not medication but deactivate or destroy virus particles, either inside or outside the body. Natural virucides are produced by some plants such as eucalyptus and Australian tea trees.

Ribavirin, also known as tribavirin, is an antiviral medication used to treat RSV infection, hepatitis C and some viral hemorrhagic fevers. For hepatitis C, it is used in combination with other medications such as simeprevir, sofosbuvir, peginterferon alfa-2b or peginterferon alfa-2a. Among the viral hemorrhagic fevers it is sometimes used for Lassa fever, Crimean–Congo hemorrhagic fever, and Hantavirus infection but should not be used for Ebola or Marburg infections. Ribavirin is taken orally or inhaled. Despite widespread usage, since the 2010s it has faced scrutiny for a lack of efficacy in treating viral infections it has historically been prescribed for.

An imidazopyridine is a nitrogen containing heterocycle that is also a class of drugs that contain this same chemical substructure. In general, they are GABAA receptor agonists, however recently proton pump inhibitors, aromatase inhibitors, NSAIDs and other classes of drugs in this class have been developed as well. Despite usually being similar to them in effect, they are not chemically related to benzodiazepines. As such, GABAA-agonizing imidazopyridines, pyrazolopyrimidines, and cyclopyrrones are sometimes grouped together and referred to as "nonbenzodiazepines." Imidazopyridines include:

Ritonavir, sold under the brand name Norvir, is an antiretroviral medication used along with other medications to treat HIV/AIDS. This combination treatment is known as highly active antiretroviral therapy (HAART). Ritonavir is a protease inhibitor, though it now mainly serves to boost the potency of other protease inhibitors. It may also be used in combination with other medications to treat hepatitis C and COVID-19. It is taken by mouth.

Rimantadine is an orally administered antiviral drug used to treat, and in rare cases prevent, influenzavirus A infection. When taken within one to two days of developing symptoms, rimantadine can shorten the duration and moderate the severity of influenza. Rimantadine can mitigate symptoms, including fever. Both rimantadine and the similar drug amantadine are derivates of adamantane. Rimantadine is found to be more effective than amantadine because when used the patient displays fewer symptoms. Rimantadine was approved by the Food and Drug Administration (FDA) in 1994.

Diphenylpyraline is a first-generation antihistamine with anticholinergic effects of the diphenylpiperidine class. It is marketed in Europe for the treatment of allergies. DPP has also been found to act as a dopamine reuptake inhibitor and produces hyperactivity in rodents. It has been shown to be useful in the treatment of Parkinsonism.

Nitazoxanide, sold under the brand name Alinia among others, is a broad-spectrum antiparasitic and broad-spectrum antiviral medication that is used in medicine for the treatment of various helminthic, protozoal, and viral infections. It is indicated for the treatment of infection by Cryptosporidium parvum and Giardia lamblia in immunocompetent individuals and has been repurposed for the treatment of influenza. Nitazoxanide has also been shown to have in vitro antiparasitic activity and clinical treatment efficacy for infections caused by other protozoa and helminths; evidence as of 2014 suggested that it possesses efficacy in treating a number of viral infections as well.
Discovery and development of nucleoside and nucleotide reverse-transcriptase inhibitors began in the 1980s when the AIDS epidemic hit Western societies. NRTIs inhibit the reverse transcriptase (RT), an enzyme that controls the replication of the genetic material of the human immunodeficiency virus (HIV). The first NRTI was zidovudine, approved by the U.S. Food and Drug Administration (FDA) in 1987, which was the first step towards treatment of HIV. Six NRTI agents and one NtRTI have followed. The NRTIs and the NtRTI are analogues of endogenous 2´-deoxy-nucleoside and nucleotide. Drug-resistant viruses are an inevitable consequence of prolonged exposure of HIV-1 to anti-HIV drugs.

Sofosbuvir, sold under the brand name Sovaldi among others, is a medication used to treat hepatitis C. It is taken by mouth.

Simeprevir, sold under the brand name Olysio among others, is a medication used in combination with other medications for the treatment of hepatitis C. It is specifically used for hepatitis C genotype 1 and 4. Medications it is used with include sofosbuvir or ribavirin and peginterferon-alfa. Cure rates are in 80s to 90s percent. It may be used in those who also have HIV/AIDS. It is taken by mouth once daily for typically 12 weeks.

Galidesivir is an antiviral drug, an adenosine analog. It was developed by BioCryst Pharmaceuticals with funding from NIAID, originally intended as a treatment for hepatitis C, but subsequently developed as a potential treatment for deadly filovirus infections such as Ebola virus disease and Marburg virus disease, as well as Zika virus. Currently, galidesivir is under phase 1 human trial in Brazil for coronavirus.

MK-608 is an antiviral drug, an adenosine analog. It was originally developed by Merck & Co. as a treatment for hepatitis C, but despite promising results in animal studies, it was ultimately unsuccessful in clinical trials. Subsequently it has been widely used in antiviral research and has shown activity against a range of viruses, including Dengue fever, tick-borne encephalitis virus, poliovirus, and most recently Zika virus, in both in vitro and animal models. Since it has already failed in human clinical trials previously, it is unlikely MK-608 itself will be developed as an antiviral medication, but the continuing lack of treatment options for these emerging viral diseases means that much research continues using MK-608 and related antiviral drugs.
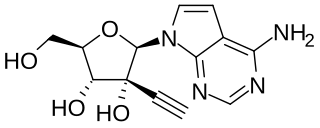
NITD008 is an antiviral drug classified as an adenosine analog. It was developed as a potential treatment for flavivirus infections and shows broad spectrum antiviral activity against many related viruses such as dengue virus, West Nile virus, yellow fever virus, Powassan virus, hepatitis C virus, Kyasanur Forest disease virus, Omsk hemorrhagic fever virus, and Zika virus. However, NITD008 proved too toxic in pre-clinical animal testing to be suitable for human trials, but it continues to be used in research to find improved treatments for emerging viral diseases.
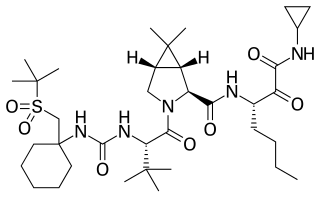
Narlaprevir, is an inhibitor of NS3/4A serine protease, intended for the treatment of chronic hepatitis C caused by genotype 1 virus in combination with other antiviral drugs.

Glecaprevir (INN,) is a hepatitis C virus (HCV) nonstructural (NS) protein 3/4A protease inhibitor that was identified jointly by AbbVie and Enanta Pharmaceuticals. It is being developed as a treatment of chronic hepatitis C infection in co-formulation with an HCV NS5A inhibitor pibrentasvir. Together they demonstrated potent antiviral activity against major HCV genotypes and high barriers to resistance in vitro.

Non-structural protein 5B (NS5B) inhibitors are a class of direct-acting antivirals widely used in the treatment of chronic hepatitis C. Depending on site of action and chemical composition, NS5B inhibitors may be categorized into three classes—nucleoside active site inhibitors (NIs), non-nucleoside allosteric inhibitors, and pyrophosphate analogues. Subsequently, all three classes are then subclassified. All inhibit RNA synthesis by NS5B but at different stages/sites resulting in inability of viral RNA replication. Expression of direct-acting NS5B inhibitors does not take place in cells that are not infected by hepatitis C virus, which seems to be beneficial for this class of drugs.

Azvudine is an antiviral drug which acts as a reverse transcriptase inhibitor. It was discovered for the treatment of hepatitis C and has since been investigated for use against other viral diseases such as AIDS and COVID-19, for which it was granted conditional approval in China.
Broad-spectrum antivirals (BSAs) are a class of molecules or compounds, which inhibit the infection of multiple viruses from the same or different virus families. BSAs could be divided into experimental and investigational agents, and approved drugs. BSAs work by inhibiting viral proteins or by targeting host cell factors and processes exploited by different viruses during infection. As of 2021, there are 150 known BSAs in varying stages of development, effective against 78 human viruses. BSAs are potential candidates for treatment of emerging and re-emerging viruses, such as ebola, marburg, and SARS-CoV-2. Many BSAs show antiviral activity against other viruses than originally investigated. Efforts in drug repurposing for SARS-CoV-2 is currently underway. A database of BSAs and viruses they inhibit could be found here.
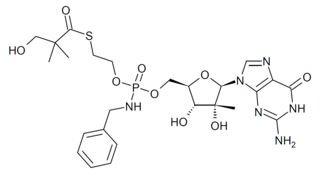
IDX-184 is an antiviral drug which was developed as a treatment for hepatitis C, acting as a NS5B RNA polymerase inhibitor. While it showed reasonable effectiveness in early clinical trials it did not progress past Phase IIb. However research using this drug has continued as it shows potentially useful activity against other emerging viral diseases such as Zika virus, and coronaviruses including MERS, and SARS-CoV-2.
Merimepodib (VX-497) is a drug which acts as an inhibitor of the enzyme inosine monophosphate dehydrogenase, which is required for the synthesis of nucleotide bases containing guanine. This consequently inhibits synthesis of DNA and RNA, and results in antiviral and immunosuppressive effects. It progressed as far as Phase 2b human clinical trials against Hepatitis C but showed only modest benefits in comparison to existing treatments, however it continues to be researched, and also shows activity against other viral diseases such as Zika virus and foot and mouth disease virus.

















