
Serotonin or 5-hydroxytryptamine (5-HT) is a monoamine neurotransmitter. Its biological function is complex, touching on diverse functions including mood, cognition, reward, learning, memory, and numerous physiological processes such as vomiting and vasoconstriction.

Tension headache, stress headache, or tension-type headache (TTH), is the most common type of primary headache. The pain usually radiates from the lower back of the head, the neck, the eyes, or other muscle groups in the body typically affecting both sides of the head. Tension-type headaches account for nearly 90% of all headaches.

5-HT receptors, 5-hydroxytryptamine receptors, or serotonin receptors, are a group of G protein-coupled receptor and ligand-gated ion channels found in the central and peripheral nervous systems. They mediate both excitatory and inhibitory neurotransmission. The serotonin receptors are activated by the neurotransmitter serotonin, which acts as their natural ligand.
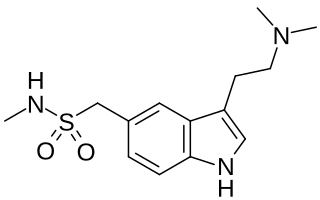
Triptans are a family of tryptamine-based drugs used as abortive medication in the treatment of migraines and cluster headaches. This drug class was first commercially introduced in the 1990s. While effective at treating individual headaches, they do not provide preventive treatment and are not considered a cure. They are not effective for the treatment of tension–type headache, except in persons who also experience migraines. Triptans do not relieve other kinds of pain.

Hyperalgesia is an abnormally increased sensitivity to pain, which may be caused by damage to nociceptors or peripheral nerves and can cause hypersensitivity to stimulus. Prostaglandins E and F are largely responsible for sensitizing the nociceptors. Temporary increased sensitivity to pain also occurs as part of sickness behavior, the evolved response to infection.

Allodynia is a condition in which pain is caused by a stimulus that does not normally elicit pain. For example, sunburn can cause temporary allodynia, so that usually painless stimuli, such as wearing clothing or running cold or warm water over it, can be very painful. It is different from hyperalgesia, an exaggerated response from a normally painful stimulus. The term comes from Ancient Greek άλλος (állos) 'other' and οδύνη (odúnē) 'pain'.
Opioid-induced hyperalgesia (OIH) or opioid-induced abnormal pain sensitivity, also called paradoxical hyperalgesia, is an uncommon condition of generalized pain caused by the long-term use of high dosages of opioids such as morphine, oxycodone, and methadone. OIH is not necessarily confined to the original affected site. This means that if the person was originally taking opioids due to lower back pain, when OIH appears, the person may experience pain in the entire body, instead of just in the lower back. Over time, individuals taking opioids can also develop an increasing sensitivity to noxious stimuli, even evolving a painful response to previously non-noxious stimuli (allodynia). This means that if the person originally felt pain from twisting or from sitting too long, the person might now additionally experience pain from a light touch or from raindrops falling on the skin.
Hyperpathia is a clinical symptom of certain neurological disorders wherein nociceptive stimuli evoke exaggerated levels of pain. This should not be confused with allodynia, where normally non-painful stimuli evoke pain.

5-Hydroxytryptamine receptor 4 is a protein that in humans is encoded by the HTR4 gene.

The 5-HT7 receptor is a member of the GPCR superfamily of cell surface receptors and is activated by the neurotransmitter serotonin (5-hydroxytryptamine, 5-HT). The 5-HT7 receptor is coupled to Gs (stimulates the production of the intracellular signaling molecule cAMP) and is expressed in a variety of human tissues, particularly in the brain, the gastrointestinal tract, and in various blood vessels. This receptor has been a drug development target for the treatment of several clinical disorders. The 5-HT7 receptor is encoded by the HTR7 gene, which in humans is transcribed into 3 different splice variants.

WAY-100635 is a piperazine drug and research chemical widely used in scientific studies. It was originally believed to act as a selective 5-HT1A receptor antagonist, but subsequent research showed that it also acts as potent full agonist at the D4 receptor. It is sometimes referred to as a silent antagonist at the former receptor. It is closely related to WAY-100135.
Mechanosensation is the transduction of mechanical stimuli into neural signals. Mechanosensation provides the basis for the senses of light touch, hearing, proprioception, and pain. Mechanoreceptors found in the skin, called cutaneous mechanoreceptors, are responsible for the sense of touch. Tiny cells in the inner ear, called hair cells, are responsible for hearing and balance. States of neuropathic pain, such as hyperalgesia and allodynia, are also directly related to mechanosensation. A wide array of elements are involved in the process of mechanosensation, many of which are still not fully understood.
SB-215505 is a drug which acts as a potent and selective antagonist at the serotonin 5-HT2B receptor, with good selectivity over the related 5-HT2A and 5-HT2C receptors. It is used in scientific research into the function of the 5-HT2 family of receptors, especially to study the role of 5-HT2B receptors in the heart, and to distinguish 5-HT2B-mediated responses from those produced by 5-HT2A or 5-HT2C.

CGS-12066A is a drug which acts as a potent and selective agonist for the 5-HT1B receptor with lower affinity for the three 5-HT2 receptor subtypes. It is used for studying the role of the 5-HT1B receptor in various processes including perception of pain and the sleep-wake cycle.

Osemozotan (MKC-242) is a selective 5-HT1A receptor agonist with some functional selectivity, acting as a full agonist at presynaptic and a partial agonist at postsynaptic 5-HT1A receptors. 5-HT1A receptor stimulation influences the release of various neurotransmitters including serotonin, dopamine, norepinephrine, and acetylcholine. 5-HT1A receptors are inhibitory G protein-coupled receptor.
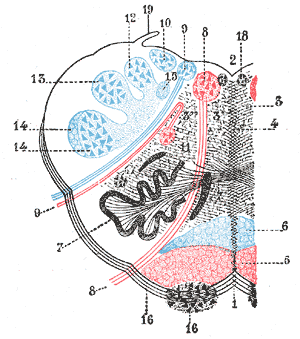
The rostral ventromedial medulla (RVM), or ventromedial nucleus of the spinal cord, is a group of neurons located close to the midline on the floor of the medulla oblongata. The rostral ventromedial medulla sends descending inhibitory and excitatory fibers to the dorsal horn spinal cord neurons. There are 3 categories of neurons in the RVM: on-cells, off-cells, and neutral cells. They are characterized by their response to nociceptive input. Off-cells show a transitory decrease in firing rate right before a nociceptive reflex, and are theorized to be inhibitory. Activation of off-cells, either by morphine or by any other means, results in antinociception. On-cells show a burst of activity immediately preceding nociceptive input, and are theorized to be contributing to the excitatory drive. Neutral cells show no response to nociceptive input.

LP-44 is a drug which acts as a potent and selective agonist at the 5HT7 serotonin receptor. While LP-44 is less selective than the related compound LP-12, it has been more widely used in research and has been used to show the complex role of 5-HT7 receptors in several aspects of brain function, including regulation of the sleep-wake cycle and roles in stress, learning and memory.

E-55888 is a drug developed by Esteve, which acts as a potent and selective full agonist at the 5HT7 serotonin receptor, and is used for investigating the role of 5-HT7 receptors in the perception of pain. When administered by itself, E-55888 is anti-hyperalgesic but not analgesic, but when administered alongside morphine, E-55888 was found to significantly increase the analgesic effects.

LP-211 is a drug which acts as a potent and selective agonist at the 5HT7 serotonin receptor, with better brain penetration than older 5-HT7 agonists in the same series, and similar effects in animals.

1-(2-Diphenyl)piperazine, also known as RA-7, is a drug which acts as a potent and selective antagonist at the 5HT7 serotonin receptor. It was discovered as an active metabolite of the synthetic 5-HT7 agonists LP-12 and LP-211, and unexpectedly turned out to be a potent antagonist with selectivity approaching that of the parent molecules, despite its much simpler structure.
















