
5-HT receptors, 5-hydroxytryptamine receptors, or serotonin receptors, are a group of G protein-coupled receptor and ligand-gated ion channels found in the central and peripheral nervous systems. They mediate both excitatory and inhibitory neurotransmission. The serotonin receptors are activated by the neurotransmitter serotonin, which acts as their natural ligand.

The 5-HT2A receptor is a subtype of the 5-HT2 receptor that belongs to the serotonin receptor family and is a G protein-coupled receptor (GPCR). The 5-HT2A receptor is a cell surface receptor, but has several intracellular locations.

A serotonin receptor agonist is an agonist of one or more serotonin receptors. They activate serotonin receptors in a manner similar to that of serotonin, a neurotransmitter and hormone and the endogenous ligand of the serotonin receptors.

The serotonin 1A receptor is a subtype of serotonin receptors, or 5-HT receptors, that binds serotonin, also known as 5-HT, a neurotransmitter. 5-HT1A is expressed in the brain, spleen, and neonatal kidney. It is a G protein-coupled receptor (GPCR), coupled to the Gi protein, and its activation in the brain mediates hyperpolarization and reduction of firing rate of the postsynaptic neuron. In humans, the serotonin 1A receptor is encoded by the HTR1A gene.

5-Hydroxytryptamine (serotonin) receptor 5A, also known as HTR5A, is a protein that in humans is encoded by the HTR5A gene. Agonists and antagonists for 5-HT receptors, as well as serotonin uptake inhibitors, present promnesic (memory-promoting) and/or anti-amnesic effects under different conditions, and 5-HT receptors are also associated with neural changes.

The 5HT6 receptor is a subtype of 5HT receptor that binds the endogenous neurotransmitter serotonin (5-hydroxytryptamine, 5HT). It is a G protein-coupled receptor (GPCR) that is coupled to Gs and mediates excitatory neurotransmission. HTR6 denotes the human gene encoding for the receptor.

The 5-HT7 receptor is a member of the GPCR superfamily of cell surface receptors and is activated by the neurotransmitter serotonin (5-hydroxytryptamine, 5-HT). The 5-HT7 receptor is coupled to Gs (stimulates the production of the intracellular signaling molecule cAMP) and is expressed in a variety of human tissues, particularly in the brain, the gastrointestinal tract, and in various blood vessels. This receptor has been a drug development target for the treatment of several clinical disorders. The 5-HT7 receptor is encoded by the HTR7 gene, which in humans is transcribed into 3 different splice variants.

5-Carboxamidotryptamine (5-CT) is a tryptamine derivative closely related to the neurotransmitter serotonin.

EMD-386088 is an indole derivative which is used in scientific research. It acts as a potent 5-HT6 receptor partial agonist, with a Ki of 1 nM, a significantly higher affinity than older 5-HT6 agonists such as EMDT, although it possesses moderate affinity for the 5-HT3 receptor as well. Subsequent research has determined that EMD-386088 is also a dopamine reuptake inhibitor and that this action is involved in the antidepressant-like effects of the drug in rodents.

SB-258585 is a drug which is used in scientific research. It acts as a potent, selective and orally active 5-HT6 receptor antagonist, with a Ki of 8.9nM. It is used in its 125I radiolabelled form to map the distribution of 5-HT6 receptors in the brain.

SB-399885 is a drug which is used in scientific research. It acts as a potent, selective and orally active 5-HT6 receptor antagonist, with a Ki of 9.0nM. SB-399885 and other 5-HT6 antagonists show nootropic effects in animal studies, as well as antidepressant and anxiolytic effects which are comparable to and synergistic with drugs such as imipramine and diazepam, and have been proposed as potential novel treatments for cognitive disorders such as schizophrenia and Alzheimer's disease.

AS-19 is a substance which acts as a potent agonist at the 5-HT7 receptor, with an IC50 of 0.83 nM. It reverses the amnesia induced by drugs such as scopolamine and dizocilpine and improves long-term memory acquisition, but inhibits short-term memory formation.
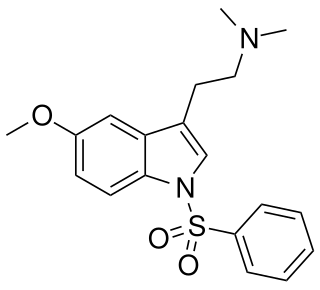
MS-245 is a tryptamine derivative used in scientific research. It acts as a selective 5-HT6 receptor antagonist with a Ki of 2.3 nM, and was derived through structure-activity relationship development of the selective 5-HT6 agonist EMDT. It has been used as a lead compound for further development of tryptamine-derived 5-HT6 antagonists. In animal studies it has been shown to boost the activity of, but not substitute for, both amphetamine and nicotine.

A serotonin releasing agent (SRA) is a type of drug that induces the release of serotonin into the neuronal synaptic cleft. A selective serotonin releasing agent (SSRA) is an SRA with less significant or no efficacy in producing neurotransmitter efflux at other types of monoamine neurons, including dopamine and norepinephrine neurons.
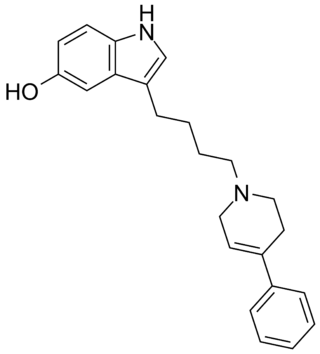
Roxindole (EMD-49,980) is a dopaminergic and serotonergic drug which was originally developed by Merck KGaA for the treatment of schizophrenia. In clinical trials its antipsychotic efficacy was only modest but it was unexpectedly found to produce potent and rapid antidepressant and anxiolytic effects. As a result, roxindole was further researched for the treatment of depression instead. It has also been investigated as a therapy for Parkinson's disease and prolactinoma.
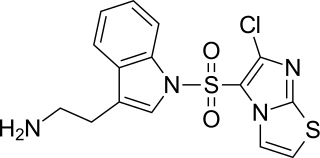
WAY-181187 is a high affinity and selective 5-HT6 receptor full agonist. It induces robust increases in extracellular GABA levels in the frontal cortex, hippocampus, striatum, and amygdala of rats without affecting concentrations in the nucleus accumbens or thalamus, and has modest to no effects on norepinephrine, serotonin, dopamine, or glutamate levels in these areas. WAY-181187 has demonstrated preclinical efficacy in rodent models of depression, anxiety, and notably obsessive-compulsive disorder, though it has also been shown to impair cognition and memory.

Pruvanserin is a selective 5-HT2A receptor antagonist which was under development by Eli Lilly and Company for the treatment of insomnia. It was in phase II clinical trials in 2008 but appears to have been discontinued as it is no longer in the company's development pipeline. In addition to its sleep-improving properties, pruvanserin has also been shown to have antidepressant, anxiolytic, and working memory-enhancing effects in animal studies.

WAY-208466 is a potent and highly selective full agonist of the 5-HT6 receptor. It increases GABA levels in the cerebral cortex and tolerance does not appear to occur to this action upon chronic administration. Animal studies have shown that WAY-208466 produces antidepressant and anxiolytic effects in rodents and it may also be useful in the treatment of obsessive-compulsive disorder.

ST-1936 (2-methyl-5-chloro-N,N-dimethyltryptamine) is a tryptamine derivative which is used in scientific research. It acts as a selective 5-HT6 receptor agonist, with a Ki of 13 nM, and much weaker action at 5-HT2B and 5-HT7 subtypes. In animal studies it has been found to increase dopamine and noradrenaline mediated signalling but decreases glutamatergic transmission, and has antidepressant effects.

Hypidone (developmental code name YL-0919) is an investigational serotonergic antidepressant which is under development for the treatment of major depressive disorder. It acts as a serotonin reuptake inhibitor, 5-HT1A receptor partial agonist, and 5-HT6 receptor full agonist. It is used as the hydrochloride salt. As of January 2021, hypidone is in phase 2 clinical trials for major depressive disorder.




















