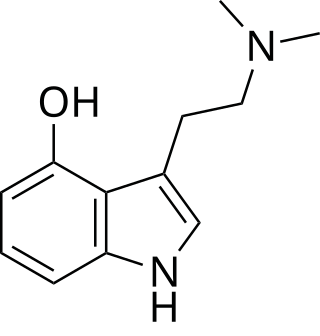
Psilocin, also known as 4-hydroxy-N,N-dimethyltryptamine (4-OH-DMT), is a substituted tryptamine alkaloid and a serotonergic psychedelic. It is present in most psychedelic mushrooms together with its phosphorylated counterpart psilocybin. Psilocin is a Schedule I drug under the Convention on Psychotropic Substances. Acting on the serotonin 5-HT2A receptors, psilocin's psychedelic effects are directly correlated with the drug's occupancy at these receptor sites. The subjective mind-altering effects of psilocin are highly variable and are said to resemble those of lysergic acid diethylamide (LSD) and N,N-dimethyltryptamine (DMT).

5-Fluoro-α-methyltryptamine, also known as PAL-212 or PAL-544, is a putative stimulant, entactogen, and psychedelic tryptamine derivative related to α-methyltryptamine (αMT).

A serotonin–dopamine releasing agent (SDRA) is a type of drug which induces the release of serotonin and dopamine in the body and/or brain.
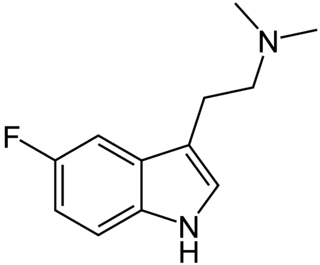
5-Fluoro-N,N-dimethyltryptamine is a tryptamine derivative related to compounds such as 5-bromo-DMT and 5-MeO-DMT. It produces a robust head-twitch response in mice, and hence is a putative serotonergic psychedelic. Fluorination of psychedelic tryptamines either reduces or has little effect on 5-HT2A/C receptor affinity or intrinsic activity, although 6-fluoro-DET is inactive as a psychedelic despite acting as a 5-HT2A agonist, while 4-fluoro-5-methoxy-DMT is a much stronger agonist at 5-HT1A than 5-HT2A.

5-Methoxy-7,N,N-trimethyltryptamine (5-MeO-7,N,N-TMT, 5-MeO-7-TMT), is a tryptamine derivative which acts as a partial agonist at the 5-HT2 serotonin receptors, with an EC50 of 63.9 nM and an efficacy of 66.2% at 5-HT2A (vs 5-HT), and weaker activity at 5-HT2B and 5-HT2C. In animal tests, both 7,N,N-TMT and 5-MeO-7,N,N-TMT produced behavioural responses similar to those of psychedelic drugs such as DMT and 5-MeO-DMT, but compounds with larger 7-position substituents such as 7-ethyl-DMT and 7-bromo-DMT did not produce psychedelic-appropriate responding despite high 5-HT2 receptor binding affinity, suggesting these may be antagonists or weak partial agonists for the 5-HT2 receptors. The related compound 7-MeO-MiPT (cf. 5-MeO-MiPT) was also found to be inactive, suggesting that the 7-position has poor tolerance for bulky groups at this position, at least if agonist activity is desired.
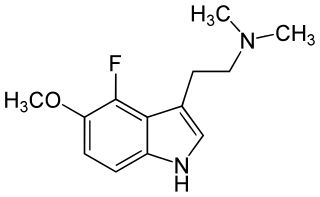
4-Fluoro-5-Methoxy-N,N-dimethyltryptamine (4-F-5-MeO-DMT) was first described by David E. Nichols team in 2000. It is a potent 5-HT1A agonist. Substitution with the 4-fluorine markedly increased 5-HT1A selectivity over 5-HT2A/2C receptors with potency greater than that of the 5-HT1A agonist 8-OH-DPAT.
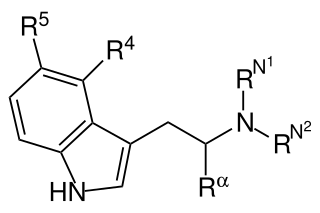
Substituted tryptamines, or simply tryptamines, also known as serotonin analogues (i.e., 5-hydroxytryptamine analogues), are organic compounds which may be thought of as being derived from tryptamine itself. The molecular structures of all tryptamines contain an indole ring, joined to an amino (NH2) group via an ethyl (−CH2–CH2−) sidechain. In substituted tryptamines, the indole ring, sidechain, and/or amino group are modified by substituting another group for one of the hydrogen (H) atoms.

O-4310 (1-isopropyl-6-fluoro-psilocin) is a tryptamine derivative developed by Organix Inc which acts as a serotonin receptor agonist. It is claimed to have an EC50 of 5 nM at the 5-HT2A receptor with 89% efficacy relative to serotonin, and 100-fold selectivity over the 5-HT2C receptor, while being apparently inactive at the 5-HT2B antitarget.

5-Chloro-α-methyltryptamine (5-Chloro-αMT), also known as PAL-542, is a tryptamine derivative related to α-methyltryptamine (αMT) and one of only a few known serotonin–dopamine releasing agents (SDRAs). It is also a potent serotonin 5-HT2A receptor agonist and hence may be a serotonergic psychedelic. The drug has been investigated in animals as a potential treatment for cocaine dependence.

5-MeO-MET (5-Methoxy-N-methyl-N-ethyltryptamine) is a relatively rare designer drug from the substituted tryptamine family, related to compounds such as N-methyl-N-ethyltryptamine and 5-MeO-DMT. It was first synthesised in the 1960s and was studied to a limited extent, but was first identified on the illicit market in June 2012 in Sweden. It was made illegal in Norway in 2013, and is controlled under analogue provisions in numerous other jurisdictions.

7-Chloro-α-methyltryptamine (7-Cl-AMT) is a tryptamine derivative with stimulant effects, invented in the 1960s. It is a weak monoamine oxidase inhibitor but its pharmacology has not otherwise been studied by modern techniques, though several closely related compounds are known to act as serotonin–dopamine releasing agents and agonists of the 5-HT2A receptor.

5-Fluoro-AET, also known as 5-fluoro-α-ethyltryptamine or by the code name PAL-545, is a substituted tryptamine derivative which acts as a serotonin–dopamine releasing agent (SDRA) and as an agonist of the serotonin 5-HT2A receptor.
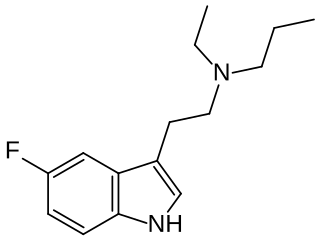
5-Fluoro-EPT (5F-EPT, 5-fluoro-N-ethyl-N-propyltryptamine) is a psychedelic tryptamine derivative related to drugs such as EPT and 5-MeO-EPT. It acts as a potent full agonist at the 5-HT2A receptor with an EC50 of 5.54 nM and an efficacy of 104% (compared to serotonin). It produces a head-twitch response in animal studies, and is claimed to have antidepressant activity.

5-Fluoro-DET is a tryptamine derivative related to drugs such as DET and 5-MeO-DET. It acts as an inhibitor of the enzyme myeloperoxidase, and is also thought to be an agonist at the 5-HT2A receptor.
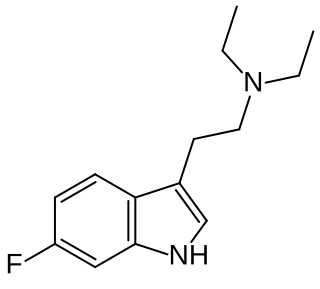
6-Fluoro-DET is a substituted tryptamine derivative related to drugs such as DET and 5-fluoro-DET. It acts as a partial agonist at the 5-HT2A receptor, but while it produces similar physiological effects to psychedelic drugs, it does not appear to produce psychedelic effects itself even at high doses. For this reason it saw some use as an active placebo in early clinical trials of psychedelic drugs but was regarded as having little use otherwise, though more recent research into compounds such as AL-34662, TBG and AAZ-A-154 has shown that these kind of non-psychedelic 5-HT2A agonists can have various useful applications.

7-F-5-MeO-MET (5-MeO-7-F-MET, 5-Methoxy-7-fluoro-N-methyl-N-ethyltryptamine) is a tryptamine derivative which acts as a serotonin receptor agonist selective for the 5-HT2 subtypes, with a pEC50 of 8.71 at 5-HT2A, vs 8.25 at 5-HT2B and 7.69 at 5-HT2C. In animal tests it produced the most potent head-twitch response of a range of related fluorinated tryptamine derivatives tested, though still with slightly lower potency than psilocybin.
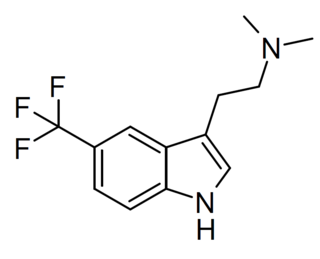
5-TFM-DMT (5-(trifluoromethyl)-DMT, 5-CF3-DMT, 5-(trifluoromethyl)-N,N-dimethyltryptamine) is a putative psychedelic tryptamine derivative related to drugs such as 5-MeO-DMT, 5-Fluoro-DMT, and 5-TFMO-DMT. It acts as a partial agonist at the 5-HT2A receptor, with an EC50 of 185.7 nM and an efficacy of 28.7% (vs 5-HT), and a full agonist at 5-HT2C with an EC50 of 89.7 nM and an efficacy of 88.8%, and no significant activity at 5-HT2B.

BK-NM-AMT, or βk-NM-αMT, also known as β-keto-N-methyl-αMT or as α,N-dimethyl-β-ketotryptamine, is a serotonin–dopamine releasing agent (SDRA) and putative entactogen of the tryptamine, α-alkyltryptamine, and β-ketotryptamine families. Along with certain other tryptamines, such as α-ethyltryptamine (αET), 5-chloro-αMT and 5-fluoro-αET, it is one of the few SDRAs known.

5-Chloro-αET, or 5-chloro-AET, also known as 5-chloro-α-ethyltryptamine, is a serotonergic agent of the tryptamine and α-alkyltryptamine families. It is the derivative of α-ethyltryptamine with a 5-chloro substitution. Analogues of 5-chloro-αET include 5-fluoro-αET, 5-chloro-αMT, and 5-fluoro-αMT.


















