Neurosteroids, also known as neuroactive steroids, are endogenous or exogenous steroids that rapidly alter neuronal excitability through interaction with ligand-gated ion channels and other cell surface receptors. The term neurosteroid was coined by the French physiologist Étienne-Émile Baulieu and refers to steroids synthesized in the brain. The term, neuroactive steroid refers to steroids that can be synthesized in the brain, or are synthesized by an endocrine gland, that then reach the brain through the bloodstream and have effects on brain function. The term neuroactive steroids was first coined in 1992 by Steven Paul and Robert Purdy. In addition to their actions on neuronal membrane receptors, some of these steroids may also exert effects on gene expression via nuclear steroid hormone receptors. Neurosteroids have a wide range of potential clinical applications from sedation to treatment of epilepsy and traumatic brain injury. Ganaxolone, a synthetic analog of the endogenous neurosteroid allopregnanolone, is under investigation for the treatment of epilepsy.

Alpidem, sold under the brand name Ananxyl, is a nonbenzodiazepine anxiolytic medication which was briefly used to treat anxiety disorders but is no longer marketed. It was previously marketed in France, but was discontinued due to liver toxicity. Alpidem is taken by mouth.

Bretazenil (Ro16-6028) is an imidazopyrrolobenzodiazepine anxiolytic drug which is derived from the benzodiazepine family, and was invented in 1988. It is most closely related in structure to the GABA antagonist flumazenil, although its effects are somewhat different. It is classified as a high-potency benzodiazepine due to its high affinity binding to benzodiazepine binding sites where it acts as a partial agonist. Its profile as a partial agonist and preclinical trial data suggests that it may have a reduced adverse effect profile. In particular bretazenil has been proposed to cause a less strong development of tolerance and withdrawal syndrome. Bretazenil differs from traditional 1,4-benzodiazepines by being a partial agonist and because it binds to α1, α2, α3, α4, α5 and α6 subunit containing GABAA receptor benzodiazepine receptor complexes. 1,4-benzodiazepines bind only to α1, α2, α3 and α5GABAA benzodiazepine receptor complexes.

The aryl hydrocarbon receptor is a protein that in humans is encoded by the AHR gene. The aryl hydrocarbon receptor is a transcription factor that regulates gene expression. It was originally thought to function primarily as a sensor of xenobiotic chemicals and also as the regulator of enzymes such as cytochrome P450s that metabolize these chemicals. The most notable of these xenobiotic chemicals are aromatic (aryl) hydrocarbons from which the receptor derives its name.
Fenobam is an imidazole derivative developed by McNeil Laboratories in the late 1970s as a novel anxiolytic drug with an at-the-time-unidentified molecular target in the brain. Subsequently, it was determined that fenobam acts as a potent and selective negative allosteric modulator of the metabotropic glutamate receptor subtype mGluR5, and it has been used as a lead compound for the development of a range of newer mGluR5 antagonists.

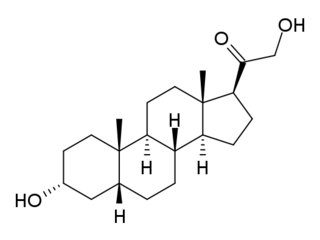
Allopregnanolone is a naturally occurring neurosteroid which is made in the body from the hormone progesterone. As a medication, allopregnanolone is referred to as brexanolone, sold under the brand name Zulresso, and used to treat postpartum depression. It is given by injection into a vein.

(+)-CPCA is a stimulant drug similar in structure to pethidine and to RTI-31, but nocaine lacks the two-carbon bridge of RTI-31's tropane skeleton. This compound was first developed as a substitute agent for cocaine.

Translocator protein (TSPO) is an 18 kDa protein mainly found on the outer mitochondrial membrane. It was first described as peripheral benzodiazepine receptor (PBR), a secondary binding site for diazepam, but subsequent research has found the receptor to be expressed throughout the body and brain. In humans, the translocator protein is encoded by the TSPO gene. It belongs to a family of tryptophan-rich sensory proteins. Regarding intramitochondrial cholesterol transport, TSPO has been proposed to interact with StAR to transport cholesterol into mitochondria, though evidence is mixed.

Imidazenil is an experimental anxiolytic drug which is derived from the benzodiazepine family, and is most closely related to other imidazobenzodiazepines such as midazolam, flumazenil, and bretazenil.

The 5-HT7 receptor is a member of the GPCR superfamily of cell surface receptors and is activated by the neurotransmitter serotonin (5-hydroxytryptamine, 5-HT). The 5-HT7 receptor is coupled to Gs (stimulates the production of the intracellular signaling molecule cAMP) and is expressed in a variety of human tissues, particularly in the brain, the gastrointestinal tract, and in various blood vessels. This receptor has been a drug development target for the treatment of several clinical disorders. The 5-HT7 receptor is encoded by the HTR7 gene, which in humans is transcribed into 3 different splice variants.

Acyl-CoA-binding protein in humans belongs to the family of Acyl-CoA-binding proteins.

IDRA-21 is a positive allosteric modulator of the AMPA receptor and a benzothiadiazine derivative. It is a chiral molecule, with (+)-IDRA-21 being the active form.

TPA-023 (MK-0777) is an anxiolytic drug with a novel chemical structure, which is used in scientific research. It has similar effects to benzodiazepine drugs, but is structurally distinct and so is classed as a nonbenzodiazepine anxiolytic. It is a mixed, subtype-selective ligand of the benzodiazepine site of α1, α2, α3, and α5-containing GABAA receptors, where it acts as a partial agonist at benzodiazepine sites of the α2 and α3-containing subtypes, but as a silent antagonist at α1 and α5-containing subtypes. It has primarily anxiolytic and anticonvulsant effects in animal tests, but with no sedative effects even at 50 times the effective anxiolytic dose.
Tetrahydrodeoxycorticosterone, also referred to as allotetrahydrocorticosterone, is an endogenous neurosteroid. It is synthesized from the adrenal hormone deoxycorticosterone by the action of two enzymes, 5α-reductase type I and 3α-hydroxysteroid dehydrogenase. THDOC is a potent positive allosteric modulator of the GABAA receptor, and has sedative, anxiolytic and anticonvulsant effects. Changes in the normal levels of this steroid particularly during pregnancy and menstruation may be involved in some types of epilepsy and premenstrual syndrome, as well as stress, anxiety and depression.

1-Amino-5-phosphonoindan-1-carboxylic acid (APICA) is a drug that is used in neuroscience research. It is a selective antagonist for the group II metabotropic glutamate receptors (mGluR2/3), and has been useful in the study of this receptor subfamily.

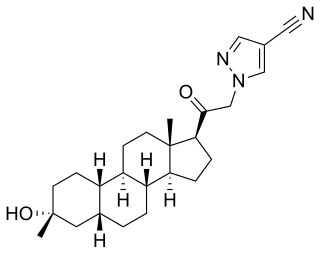
Emapunil is an anxiolytic drug which acts as a selective agonist at the peripheral benzodiazepine receptor, also known as the mitochondrial 18 kDa translocator protein or TSPO. This protein has multiple functions, among which is regulation of steroidogenesis, particularly the production of neuroactive steroids such as allopregnanolone in the brain. In both animal and human trials, emapunil produced fast acting anxiolytic and anti-panic effects, without producing sedation or withdrawal symptoms following cessation of use. Emapunil is also used in its 11C radiolabelled form to map the distribution of TSPO receptors in the brain.

FGIN-1-27 is an anxiolytic drug which acts as a selective agonist at the peripheral benzodiazepine receptor, also known as the mitochondrial 18 kDa translocator protein or TSPO. It is thought to produce anxiolytic effects by stimulating steroidogenesis of neuroactive steroids such as allopregnanolone.
Sazetidine A (AMOP-H-OH) is a drug which acts as a subtype selective partial agonist at α4β2 neural nicotinic acetylcholine receptors, acting as an agonist at (α4)2(β2)3 pentamers, but as an antagonist at (α4)3(β2)2 pentamers. It has potent analgesic effects in animal studies comparable to those of epibatidine, but with less toxicity, and also has antidepressant action.

Adatanserin is a mixed 5-HT1A receptor partial agonist and 5-HT2A and 5-HT2C receptor antagonist. It was under development by Wyeth as an antidepressant but was ultimately not pursued.

Vassilios Papadopoulos is a Greek scholar, researcher, inventor, professor, and university administrator who has served as dean of the USC Alfred E. Mann School of Pharmacy and Pharmaceutical Sciences at the University of Southern California in Los Angeles, California, since 2016. Previously, he was the associate vice president and director of the Biomedical Graduate Research Organization at Georgetown University from 2005 to 2007, and the executive director and chief scientific officer of the Research Institute of the McGill University Health Center from 2007 to 2015.



















