 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Afobazole |
| Other names | Obenoxazine |
| Routes of administration | Oral |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 43.64%, pronounced first-pass effect |
| Metabolism | extensive hepatic |
| Onset of action | 0.85±0.13 hours |
| Elimination half-life | 0.82±0.54 hours |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
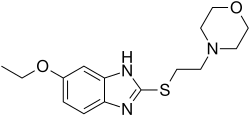
| Formula | C15H21N3O2S |
| Molar mass | 307.41 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |

Fabomotizole (INN; [1] brand name Afobazole) is an anxiolytic drug launched in Russia in the early 2000s. It produces anxiolytic and neuroprotective effects without any sedative or muscle relaxant actions.[ citation needed ] Its mechanism of action remains poorly defined however, with GABAergic, NGF- and BDNF-release-promoting, MT1 receptor agonism, MT3 receptor antagonism, and sigma agonism suggested as potential mechanisms. Fabomotizole was shown to inhibit MAO-A reversibly and there might be also some involvement with serotonin receptors. [2] [3] [4] [5] [6] Clinical trials have shown fabomotizole to be well tolerated and reasonably effective for the treatment of anxiety. [7]
Experiments in mice have shown antimutagenic and antiteratogenic properties. [8]
Experiments in rats have shown beneficial effect in the model of ischemic stroke. [9]
Fabomotizole has found little clinical use outside Russia and has not been evaluated by the FDA.