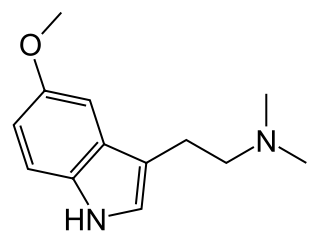
5-MeO-DMT (5-methoxy-N,N-dimethyltryptamine) or O-methyl-bufotenin is a psychedelic of the tryptamine class. It is found in a wide variety of plant species, and also is secreted by the glands of at least one toad species, the Colorado River toad. Like its close relatives DMT and bufotenin (5-HO-DMT), it has been used as an entheogen in South America. Slang terms include Five-methoxy, the power, bufo, and toad venom.
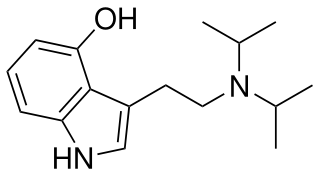
4-Hydroxy-N,N-diisopropyltryptamine is a synthetic psychedelic drug. It is a higher homologue of psilocin, 4-HO-DET, and is a positional isomer of 4-HO-DPT and has a tryptamine molecular sub-structure.
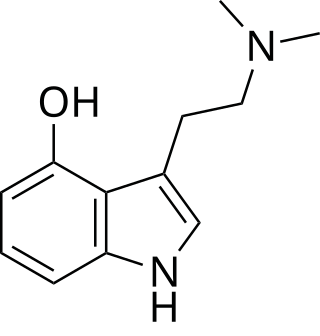
Psilocin is a substituted tryptamine alkaloid and a serotonergic psychedelic substance. It is present in most psychedelic mushrooms together with its phosphorylated counterpart psilocybin. Psilocin is a Schedule I drug under the Convention on Psychotropic Substances. Acting on the 5-HT2A receptors, psilocin modulates the production and reuptake of serotonin. The mind-altering effects of psilocin are highly variable and subjective and resemble those of LSD and DMT.

Diisopropyltryptamine is a psychedelic hallucinogenic drug of the tryptamine family that has a unique effect. While the majority of hallucinogens affect the visual sense, DiPT is primarily aural.

DET, also known under its chemical name N,N-diethyltryptamine and as T-9, is a psychedelic drug closely related to DMT and 4-HO-DET. However, despite its structural similarity to DMT, its activity is induced by an oral dose of around 50–100 mg, without the aid of MAO inhibitors, and the effects last for about 2–4 hours.
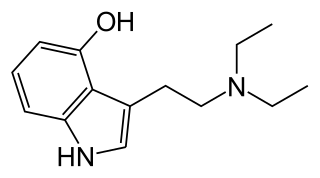
4-HO-DET, also known as 4-hydroxy-diethyl-tryptamine, CZ-74, is a hallucinogenic drug and psychedelic compound of moderate duration. 4-HO-DET is a substituted tryptamine, structurally related to psilocin, ethocybin, and 4-HO-DIPT.

5-MeO-MiPT is a psychedelic and hallucinogenic drug, used by some as an entheogen. It has structural and pharmacodynamic properties similar to the drugs 5-MeO-DiPT, DiPT, and MiPT. It is commonly used as a "substitute" for 5-MeO-DiPT because of the very similar structure and effects.

5-MeO-DET or 5-methoxy-N,N-diethyltryptamine is a hallucinogenic tryptamine.

4-HO-MiPT is a synthetic substituted aromatic compound and a lesser-known psychedelic tryptamine. It is thought to be a serotonergic psychedelic, similar to magic mushrooms, LSD and mescaline. Its molecular structure and pharmacological effects somewhat resemble those of the tryptamine psilocin, which is the primary psychoactive chemical in magic mushrooms.

N,N-Dibutyltryptamine (DBT) is a psychedelic drug belonging to the tryptamine family. It is found either as its crystalline hydrochloride salt or as an oily or crystalline base. DBT was first synthesized by the chemist Alexander Shulgin and reported in his book TiHKAL . Shulgin did not test DBT himself, but reports a human dosage of "1 mg/kg i.m." being active, but less so than DMT or DET. This suggests that an active dosage of DBT will be in the 100 mg range. This compound has been sold as a "research chemical" and has been confirmed to be an active hallucinogen although somewhat weaker than other similar tryptamine derivatives. It produces a head-twitch response in mice.

4-MeO-MiPT, or 4-methoxy-N-methyl-N-isopropyltryptamine, is a lesser-known psychedelic drug. It is the 4-methoxy analog of MiPT. 4-MeO-MiPT was first synthesized by Alexander Shulgin and is mentioned in his book TiHKAL. Subsequent testing by Shulgin on human test subjects showed the effective dose as 20-30 mg ; the onset time between ingestion and the first noticeable effects was 45-60 min, with sensations lasting between 2-2.5 hours. The sensation were significantly milder than those of 4-HO-MiPT, with 4-MeO-MiPT producing erotic-enhancing effects, and few of the visuals common with tryptamines. Very little data exists about the pharmacological properties, metabolism, and toxicity of 4-MeO-MiPT.
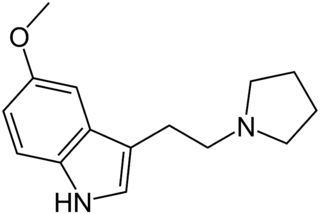
5-MeO-pyr-T (5-methoxy-N,N-tetramethylenetryptamine) is a lesser-known psychedelic drug. It is the 5-methoxy analog of pyr-T. 5-MeO-pyr-T was first synthesized by Hunt & Brimblecombe, who credited S. Mitzal for characterization of chemical properties. Later human tests were reported by Alexander Shulgin, in his book TiHKAL. An oral dosage of 0.5 to 2 mg, and an inhaled dosage of 2–3 mg are reported. 5-MeO-pyr-T causes varying reactions, such as amnesia, tinnitus, vomiting, and a 5-MeO-DMT-like rushing sensation. At the highest dosage reported in TiHKAL, the subject describes awakening from an apparent fugue state during which they were wandering the streets, with complete amnesia upon awakening.
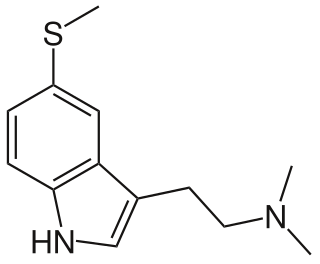
5-MeS-DMT (5-methylthio-N,N-dimethyltryptamine) is a lesser-known psychedelic drug. It is the 5-methylthio analog of dimethyltryptamine (DMT). 5-MeS-DMT was first synthesized by Alexander Shulgin. In his book TiHKAL, the minimum dosage is listed as 15-30 mg. The duration listed as very short, just like DMT. 5-MeS-DMT produces similar effects to DMT, but weaker. Shulgin describes his feelings while on a low dose of this drug as "pointlessly stoned", although at a higher dose of 20 mg he says it is "quite intense" and suggests that a higher dose still might have full activity.
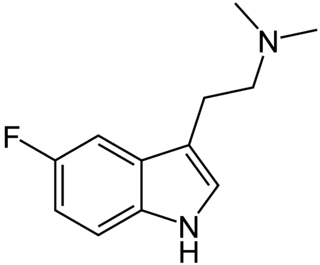
5-Fluoro-N,N-dimethyltryptamine is a tryptamine derivative related to compounds such as 5-bromo-DMT and 5-MeO-DMT. Fluorination of psychedelic tryptamines either reduces or has little effect on 5-HT2A/C receptor affinity or intrinsic activity, although 6-fluoro-DET is inactive as a psychedelic despite acting as a 5-HT2A agonist, while 4-fluoro-5-methoxy-DMT is a much stronger agonist at 5-HT1A than 5-HT2A.

4-Hydroxy-α-methyltryptamine (4-HO-αMT) is a psychedelic drug of the tryptamine class. It is a close structural analogue of α-methyltryptamine (αMT) and produces similar effects to it, but with exacerbated side effects similarly to 5-MeO-αMT. Alexander Shulgin describes 4-HO-αMT briefly in his book TiHKAL:
The 4-hydroxy analogue of αMT has been looked at in human subjects. It is reported to be markedly visual in its effects, with some subjects reporting dizziness and a depressed feeling. There were, however, several toxic signs at doses of 15 to 20 milligrams orally, including abdominal pain, tachycardia, increased blood pressure and, with several people, headache and diarrhea.
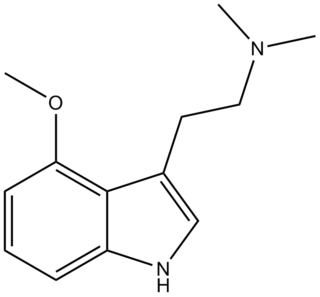
4-MeO-DMT (4-methoxy-N,N-dimethyltryptamine) is a tryptamine derivative which has some central activity in animal tests similar to that of related psychedelic tryptamine drugs, although with significantly lower potency than either 5-MeO-DMT or 4-hydroxy-DMT (psilocin).

5-MeO-MET (5-Methoxy-N-methyl-N-ethyltryptamine) is a relatively rare designer drug from the substituted tryptamine family, related to compounds such as N-methyl-N-ethyltryptamine and 5-MeO-DMT. It was first synthesised in the 1960s and was studied to a limited extent, but was first identified on the illicit market in June 2012 in Sweden. It was made illegal in Norway in 2013, and is controlled under analogue provisions in numerous other jurisdictions.
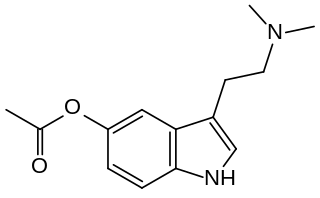
O-Acetylbufotenine is a tryptamine derivative which produces psychedelic-appropriate responding in animal studies. It is an acylated derivative of bufotenine with higher lipophilicity that allows it to cross the blood–brain barrier; once inside the brain, it is metabolised to bufotenine. It also acts directly as an agonist at 5-HT1A and 5-HT1D receptors.


















