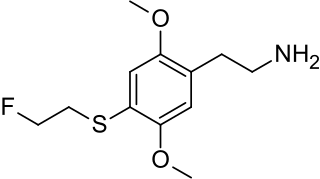
2C-T-21 is a psychedelic phenethylamine of the 2C family sometimes used as an entheogen. It was first synthesized by Alexander Shulgin.

3C-P (α-Methyl-4-propoxy-3,5-dimethoxyphenethylamine) is a psychedelic phenethylamine. It has structural and pharmacodynamic properties similar to the drugs mescaline, proscaline, and amphetamine. Little information exists on the human pharmacology of 3C-P, but a psychedelic dosage appears to be 20–40 mg, and is accompanied by stimulant and psychedelic effects such as visual enhancement and distortion. It can be synthesized from syringaldehyde by reaction with n-propyl iodide followed by condensation with nitroethane and reduction.
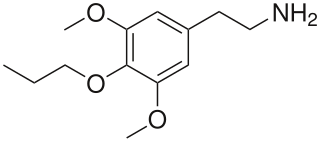
Proscaline is a psychedelic and hallucinogenic drug. It has structural properties similar to the drugs mescaline, isoproscaline, and escaline. In PiHKAL, Alexander Shulgin reports that a dose of 30–60 mg produces effects lasting 8–12 hours.

2C-TFM is a psychedelic phenethylamine of the 2C family. It was first synthesized in the laboratory of David E. Nichols. It has also been called 2C-CF3, a name derived from the Para-trifluoromethyl group it contains.
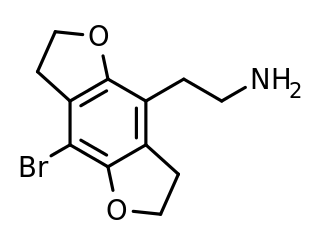
2C-B-FLY is a psychedelic phenethylamine and designer drug of the 2C family. It was first synthesized in 1996 by Aaron Monte, Professor of Chemistry at UW-La Crosse.
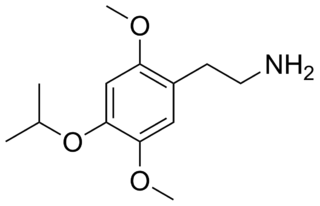
2C-O-4 (4-isopropoxy-2,5-dimethoxyphenethylamine) is a phenethylamine of the 2C family. It is also a positional isomer of isoproscaline and was probably first synthesized by Alexander Shulgin. It produces hallucinogenic, psychedelic, and entheogenic effects. Because of the low potency of 2C-O-4, and the inactivity of 2C-O, Shulgin felt that the 2C-O series would not be an exciting area for research, and did not pursue any further analogues.

2C-H (2,5-dimethoxyphenethylamine) is a lesser-known substituted phenethylamine of the 2C family.

Allylescaline (4-allyloxy-3,5-dimethoxyphenethylamine) is a lesser-known psychedelic drug. It is closely related in structure to mescaline. Allylescaline was first synthesized by Otakar Leminger in 1972. The compound was later synthesized by Alexander Shulgin and further described in his book PiHKAL. The dosage range is listed as 20–35 mg, and the duration 8–12 hours. Allylescaline produces an entactogenic warmth, an entheogenic effect, and a feeling of flowing energy. Very little data exists about the pharmacological properties, metabolism, and toxicity of allylescaline.

Methallylescaline (4-Methylallyloxy-3,5-dimethoxyphenethylamine) is a lesser-known psychedelic drug. It is the 4-methyl analog of allylescaline. Methallylescaline was first synthesized by Alexander Shulgin. In his book PiHKAL, the dosage range is listed as 40–65 mg and the duration is listed as 12–16 hours. Little data exists about the pharmacological properties, metabolism, and toxicity of methallylescaline, though it is known to be an agonist of 5-HT2A receptors, with effects comparable to that of other mescaline analogs and has been sold as a designer drug.

2C-YN is an analog of phenethylamine that can be synthesized from 2C-I. Very little data exists about the pharmacological properties, metabolism, and toxicity of 2C-YN, although Daniel Trachsel lists it as having a dosage of around 50mg and a duration of around 2 hours, with relatively mild psychedelic effects.

25B-NBOMe is a derivative of the phenethylamine psychedelic 2C-B, discovered in 2004 by Ralf Heim at the Free University of Berlin. It acts as a potent full agonist for the 5HT2A receptor. Anecdotal reports from users suggest 25B-NBOMe to be an active hallucinogen at a dose of as little as 250–500 µg, making it a similar potency to other phenethylamine derived hallucinogens such as Bromo-DragonFLY. Duration of effects lasts about 12–16 hours, although the parent compound is rapidly cleared from the blood when used in the radiolabeled form in tracer doses. Recently, Custodio et al (2019) evaluated the potential involvement of dysregulated dopaminergic system, neuroadaptation, and brain wave changes which may contribute to the rewarding and reinforcing properties of 25B-NBOMe in rodents.
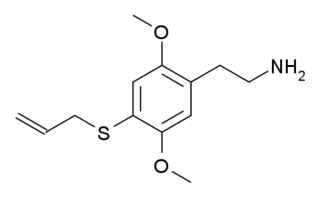
2C-T-16 is a lesser-known psychedelic drug. It was originally named by Alexander Shulgin as described in his book PiHKAL, however while Shulgin began synthesis of this compound he only got as far as the nitrostyrene intermediate, and did not complete the final synthetic step. Synthesis of 2C-T-16 was finally achieved by Daniel Trachsel some years later, and it was subsequently reported as showing similar psychedelic activity to related compounds, with a dose range of 10–25 mg and a duration of 4–6 hours, making it around the same potency as the better-known saturated analogue 2C-T-7, but with a significantly shorter duration of action. Binding studies in vitro showed 2C-T-16 to have a binding affinity of 44 nM at 5-HT2A and 15 nM at 5-HT2C. 2C-T-16 and related derivatives are potent partial agonists of the 5-HT1A, 5-HT2A, 5-HT2B and 5-HT2C receptors and induce a head-twitch response in mice.
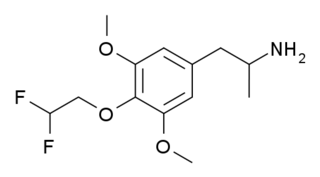
3C-DFE is a lesser-known psychedelic drug, which is a fluorinated derivative of 3C-E. It was first synthesised by Daniel Trachsel in 2002, and has been reported as showing similar psychedelic activity to related compounds, with a dose range of around 20–40 mg and a duration of approximately 10 hours. Despite its reported psychedelic activity, binding studies in vitro showed 3C-DFE to have a surprisingly weak binding affinity of 2695 nM at 5-HT2A with negligible affinity at 5-HT2C, making it only slightly higher affinity than mescaline, despite its higher potency in vivo.

Trifluoromescaline (TF-M) is a derivative of the phenethylamine hallucinogen mescaline, which has a trifluoromethoxy group replacing the central methoxy group of mescaline. Synthesis of this compound was first reported by Daniel Trachsel in 2011, alongside many other related compounds. Trifluoromescaline was found to be one of the most potent compounds in the series, with a reported dosage of 15–40 mg, and a slow onset of action and long duration of effects, lasting 14–24 hours or more.

2C-TFE is a lesser-known psychedelic drug related to compounds such as 2C-E and 2C-TFM. It was first synthesised by Daniel Trachsel, and is reportedly a potent psychedelic with an active dose in the 5–15 mg range, and a long duration of action of 12–24 hours.

2C-T-3 is a lesser-known psychedelic drug related to compounds such as 2C-T-7 and 2C-T-16. It was named by Alexander Shulgin but was never made or tested by him, and was instead first synthesised by Daniel Trachsel some years later. It has a binding affinity of 11nM at 5-HT2A and 40nM at 5-HT2C. It is reportedly a potent psychedelic drug with an active dose in the 15–40 mg range, and a duration of action of 8–14 hours, with visual effects comparable to related drugs such as methallylescaline.

3C-MAL (4-Methylallyloxy-3,5-dimethoxyamphetamine) is a psychedelic phenethylamine with structural similarities to methallylescaline. Little information exists on the human pharmacology of 3C-MAL and it has little-to-no history of human use. The hydrochloride salt is a white crystal with a melting point of 159–160 °C (318–320 °F).

2C-T-28 is a lesser-known psychedelic drug related to compounds such as 2C-T-7 and 2C-T-21. It was named by Alexander Shulgin but was never made or tested by him, and was instead first synthesised by Daniel Trachsel some years later. It has a binding affinity of 75 nM at 5-HT2A and 28 nM at 5-HT2C. It is reportedly a potent psychedelic drug with an active dose in the 8–20 mg range, and a duration of action of 8–10 hours, with prominent visual effects. 2C-T-28 is the 3-fluoropropyl instead of 2-fluoroethyl chain-lengthened homologue of 2C-T-21 and has very similar properties, although unlike 2C-T-21 it will not form toxic fluoroacetate as a metabolite.
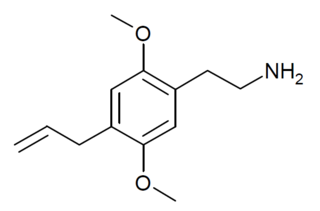
2C-AL is a drug from the substituted phenethylamine family which acts as an agonist of the 5-HT2A receptor, with an EC50 of 2.15nM at 5-HT2A vs 77.71nM at 5-HT2B, and produces a head-twitch response in animal studies. It was first discussed as a hypothetical compound in Daniel Trachsel's 2013 review of the field after his successful synthesis of the related compounds 2C-V and 2C-YN, and finally synthesised by a team at Gilgamesh Pharmaceuticals in 2020 using a different synthetic route from that employed by Trachsel.



















