Ephedrine is a central nervous system (CNS) stimulant that is often used to prevent low blood pressure during anesthesia. It has also been used for asthma, narcolepsy, and obesity but is not the preferred treatment. It is of unclear benefit in nasal congestion. It can be taken by mouth or by injection into a muscle, vein, or just under the skin. Onset with intravenous use is fast, while injection into a muscle can take 20 minutes, and by mouth can take an hour for effect. When given by injection it lasts about an hour and when taken by mouth it can last up to four hours.

Benzphetamine is a substituted amphetamine used short-term along with a doctor-approved, reduced-calorie diet, exercise, and behavioral program for weight loss. It is prescribed for obesity to people who have been unable to lose weight through exercise and dieting alone. It is a prodrug to dextroamphetamine and dextromethamphetamine.

D-norpseudoephedrine, also known as cathine and (+)-norpseudoephedrine, is a psychoactive drug of the phenethylamine and amphetamine chemical classes which acts as a stimulant. Along with cathinone, it is found naturally in Catha edulis (khat), and contributes to its overall effects. It has approximately 7-10% the potency of amphetamine.

Fenfluramine, sold under the brand name Fintepla, is a serotonergic medication used for the treatment of seizures associated with Dravet syndrome and Lennox–Gastaut syndrome. It was formerly used as an appetite suppressant in the treatment of obesity, but was discontinued for this use due to cardiovascular toxicity before being repurposed for new indications. Fenfluramine was used for weight loss both alone under the brand name Pondimin and in combination with phentermine.
Anti-obesity medication or weight loss medications are pharmacological agents that reduce or control excess body fat. These medications alter one of the fundamental processes of the human body, weight regulation, by reducing appetite and consequently energy intake, increasing energy expenditure, redirecting nutrients from adipose to lean tissue, or interfering with the absorption of calories.

Phentermine (phenyl-tertiary-butylamine), with several brand names including Ionamin and Sentis, is a medication used together with diet and exercise to treat obesity. It is taken by mouth for up to a few weeks at a time, after which the benefits subside. It is also available as the combination phentermine/topiramate.

Sibutramine, formerly sold under the brand name Meridia among others, is an appetite suppressant which has been discontinued in many countries. It works as a serotonin–norepinephrine reuptake inhibitor similar to a tricyclic antidepressant. Until 2010, it was widely marketed and prescribed as an adjunct in the treatment of obesity along with diet and exercise. It has been associated with increased cardiovascular diseases and strokes and has been withdrawn from the market in 2010 in several countries and regions including Australia, Canada, China, the European Union, Hong Kong, India, Mexico, New Zealand, the Philippines, Thailand, the United Kingdom, and the United States. However, the drug remains available in some countries.

Phenmetrazine is a stimulant drug first synthesized in 1952 and originally used as an appetite suppressant, but withdrawn from the market in the 1980s due to widespread abuse. It was initially replaced by its analogue phendimetrazine which functions as a prodrug to phenmetrazine, but now it is rarely prescribed, due to concerns of abuse and addiction. Chemically, phenmetrazine is a substituted amphetamine containing a morpholine ring.

Armodafinil (trade name Nuvigil) is the enantiopure compound of the eugeroic modafinil (Provigil). It consists of only the (R)-(−)-enantiomer of the racemic modafinil. Armodafinil is produced by the pharmaceutical company Cephalon Inc. and was approved by the U.S. Food and Drug Administration (FDA) in June 2007. In 2016, the FDA granted Mylan rights for the first generic version of Cephalon's Nuvigil to be marketed in the U.S.

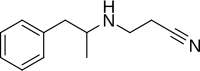
Amfepramone, also known as diethylpropion, is a stimulant drug of the phenethylamine, amphetamine, and cathinone classes that is used as an appetite suppressant. It is used in the short-term management of obesity, along with dietary and lifestyle changes. Amfepramone has a similar chemical structure to the antidepressant and smoking cessation aid bupropion, which has also been developed as a weight-loss medicine when in a combination product with naltrexone.

Aminorex is a weight loss (anorectic) stimulant drug. It was withdrawn from the market after it was found to cause pulmonary hypertension. In the U.S., it is an illegal Schedule I drug, meaning it has high abuse potential, no accepted medical use, and a poor safety profile.

Dexfenfluramine, marketed as dexfenfluramine hydrochloride under the name Redux, is a serotonergic anorectic drug: it reduces appetite by increasing the amount of extracellular serotonin in the brain. It is the d-enantiomer of fenfluramine and is structurally similar to amphetamine, but lacks any psychologically stimulating effects.

Clobenzorex is a stimulant drug of the amphetamine chemical class used as an appetite suppressant. The drug is legally distributed in Mexico under the trade name Asenlix by Aventis.

β-Methylphenethylamine is an organic compound of the phenethylamine class, and a positional isomer of the drug amphetamine, with which it shares some properties. In particular, both amphetamine and β-methylphenethylamine are human TAAR1 agonists. In appearance, it is a colorless or yellowish liquid.

Etilamfetamine is a stimulant drug of the phenethylamine and amphetamine chemical classes. It was invented in the early 20th century and was subsequently used as an anorectic or appetite suppressant in the 1950s, but was not as commonly used as other amphetamines such as amphetamine, methamphetamine, and benzphetamine, and was largely discontinued once newer drugs such as phenmetrazine were introduced. It most likely acts primarily as a dopamine releasing agent. Its activity as a norepinephrine or serotonin releasing agent is not known.
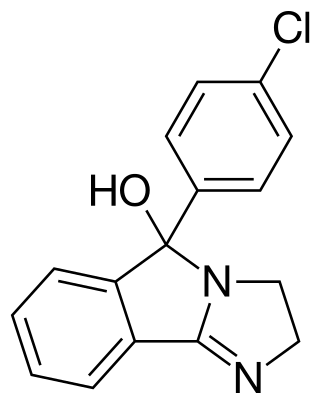
Mazindol is a stimulant drug which is used as an appetite suppressant. It was developed by Sandoz-Wander in the 1960s.

Mefenorex is a stimulant drug which was used as an appetite suppressant. It is an amphetamine derivative which was developed in the 1970s and used for the treatment of obesity. Mefenorex produces amphetamine as a metabolite, and has been withdrawn in many countries despite having only mild stimulant effects and relatively little abuse potential.

Methylhexanamine is an indirect sympathomimetic drug invented and developed by Eli Lilly and Company and marketed as an inhaled nasal decongestant from 1948 until it was voluntarily withdrawn from the market in the 1980s.
Substituted amphetamines are a class of compounds based upon the amphetamine structure; it includes all derivative compounds which are formed by replacing, or substituting, one or more hydrogen atoms in the amphetamine core structure with substituents. The compounds in this class span a variety of pharmacological subclasses, including stimulants, empathogens, and hallucinogens, among others. Examples of substituted amphetamines are amphetamine (itself), methamphetamine, ephedrine, cathinone, phentermine, mephentermine, tranylcypromine, bupropion, methoxyphenamine, selegiline, amfepramone (diethylpropion), pyrovalerone, MDMA (ecstasy), and DOM (STP).
Oxilofrine is a substituted phenethylamine stimulant drug chemically related to ephedrine and to synephrine.