
Modafinil, sold under the brand name Provigil among others, is a wakefulness-promoting medication used primarily to treat narcolepsy, a sleep disorder characterized by excessive daytime sleepiness and sudden sleep attacks. Modafinil is also approved for stimulating wakefulness in people with sleep apnea and shift work sleep disorder. It is taken by mouth. Modafinil is not approved by the US Food and Drug Administration (FDA) for use in people under 17 years old.
A dopamine reuptake inhibitor (DRI) is a class of drug which acts as a reuptake inhibitor of the monoamine neurotransmitter dopamine by blocking the action of the dopamine transporter (DAT). Reuptake inhibition is achieved when extracellular dopamine not absorbed by the postsynaptic neuron is blocked from re-entering the presynaptic neuron. This results in increased extracellular concentrations of dopamine and increase in dopaminergic neurotransmission.
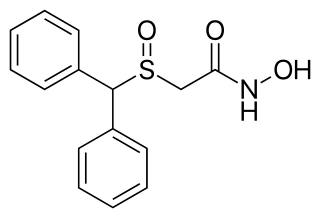
Adrafinil, sold under the brand name Olmifon, is a wakefulness-promoting medication that was formerly used in France to improve alertness, attention, wakefulness, and mood, particularly in the elderly. It was also used off-label by individuals who wished to avoid fatigue, such as night workers or others who needed to stay awake and alert for long periods of time. Additionally, the medication has been used non-medically as a novel vigilance-promoting agent.

Dopaminergic means "related to dopamine", dopamine being a common neurotransmitter. Dopaminergic substances or actions increase dopamine-related activity in the brain.

Armodafinil (trade name Nuvigil) is the enantiopure compound of the eugeroic modafinil (Provigil). It consists of only the (R)-(−)-enantiomer of the racemic modafinil. Armodafinil is produced by the pharmaceutical company Cephalon Inc. and was approved by the U.S. Food and Drug Administration (FDA) in June 2007. In 2016, the FDA granted Mylan rights for the first generic version of Cephalon's Nuvigil to be marketed in the U.S.

(+)-CPCA is a stimulant drug similar in structure to pethidine and to RTI-31, but nocaine lacks the two-carbon bridge of RTI-31's tropane skeleton. This compound was first developed as a substitute agent for cocaine.

Troparil is a stimulant drug used in scientific research. Troparil is a phenyltropane-based dopamine reuptake inhibitor (DRI) that is derived from methylecgonidine. Troparil is a few times more potent than cocaine as a dopamine reuptake inhibitor, but is less potent as a serotonin reuptake inhibitor, and has a duration spanning a few times longer, since the phenyl ring is directly connected to the tropane ring through a non-hydrolyzable carbon-carbon bond. The lack of an ester linkage removes the local anesthetic action from the drug, so troparil is a pure stimulant. This change in activity also makes troparil slightly less cardiotoxic than cocaine. The most commonly used form of troparil is the tartrate salt, but the hydrochloride and naphthalenedisulfonate salts are also available, as well as the free base.
Difluoropine (O-620) is a stimulant drug synthesised from tropinone, which acts as a potent and selective dopamine reuptake inhibitor. Difluoropine is unique among the tropane-derived dopamine reuptake inhibitors in that the active stereoisomer is the (S) enantiomer rather than the (R) enantiomer, the opposite way round compared to natural cocaine. It is structurally related to benztropine and has similar anticholinergic and antihistamine effects in addition to its dopamine reuptake inhibitory action.

Narcolepsy is a chronic neurological disorder that impairs the ability to regulate sleep–wake cycles, and specifically impacts REM sleep. The pentad symptoms of narcolepsy include excessive daytime sleepiness (EDS), sleep related hallucinations, sleep paralysis, disturbed nocturnal sleep (DNS) and cataplexy. There are two recognized forms of narcolepsy, narcolepsy type 1 and type 2. Narcolepsy type 1 (NT1) can be clinically characterized by symptoms of EDS and cataplexy, and/or will have CSF orexin levels of less than 110 pg/ml. Cataplexy are transient episodes of aberrant tone, most typically loss of tone, that can be associated with strong emotion. In pediatric onset narcolepsy, active motor phenomena are not uncommon. Cataplexy may be mistaken for syncope, tic disorder or seizures. Narcolepsy type 2 (NT2) does not have features of cataplexy and CSF orexin levels are normal. Sleep related hallucinations, also known as hypnogogic and hypnopompic are vivid hallucinations that can be auditory, visual or tactile and may occur independent of or in combination with an inability to move. People with narcolepsy tend to sleep about the same number of hours per day as people without it, but the quality of sleep is typically compromised. Narcolepsy is a clinical syndrome of hypothalamic disorder, but the exact cause of narcolepsy is unknown, with potentially several causes. A leading consideration for the cause of narcolepsy type 1 is that it is an autoimmune disorder. Proposed pathophysiology as an autoimmune disease suggest antigen presentation by DQ0602 to specific CD4+ T cells resulting in CD8+ T-cell activation and consequent injury to orexin producing neurons. Familial trends of narcolepsy are suggested to be higher than previously appreciated. Familial risk of narcolepsy among first degree relatives is high. Relative risk for narcolepsy in a first degree relative has been reported to be 361.8. However, it is important to note that there is a spectrum of symptoms found in this study, including asymptomatic abnormal sleep test findings to significantly symptomatic.
Tropoxane (O-1072) is an aryloxytropane derivative drug developed by Organix Inc., which acts as a stimulant and potent dopamine and serotonin reuptake inhibitor. It is an analogue of dichloropane where the amine nitrogen has been replaced by an oxygen ether link, demonstrating that the amine nitrogen is not required for DAT binding and reuptake inhibition.
A dopamine releasing agent (DRA) is a type of drug which induces the release of dopamine in the body and/or brain. No selective and robust DRAs are currently known. On the other hand, many releasing agents of both dopamine and norepinephrine and of serotonin, norepinephrine, and dopamine are known. Serotonin–dopamine releasing agents (SDRAs), for instance 5-chloro-αMT, are much more rare and are not selective for dopamine release but have also been developed. Examples of major NDRAs include the psychostimulants amphetamine and methamphetamine, while an example of an SNDRA is the entactogen methylenedioxymethamphetamine (MDMA). These drugs are frequently used for recreational purposes and encountered as drugs of abuse. Selective DRAs, as well as NDRAs, have medical applications in the treatment of attention deficit hyperactivity disorder (ADHD).

Eugeroics, also known as wakefulness-promoting agents and wakefulness-promoting drugs, are a class of drugs that promote wakefulness and alertness. They are medically indicated for the treatment of certain sleep disorders including excessive daytime sleepiness (EDS) in narcolepsy or obstructive sleep apnea (OSA). Eugeroics are also often prescribed off-label for the treatment of EDS in idiopathic hypersomnia. In contrast to classical psychostimulants, such as methylphenidate and amphetamine, which are also used in the treatment of these disorders, eugeroics typically do not produce marked euphoria, and, consequently, have a lower addictive potential.

A norepinephrine–dopamine reuptake inhibitor (NDRI) is a drug used for the treatment of clinical depression, attention deficit hyperactivity disorder (ADHD), narcolepsy, and the management of Parkinson's disease. The drug acts as a reuptake inhibitor for the neurotransmitters norepinephrine and dopamine by blocking the action of the norepinephrine transporter (NET) and the dopamine transporter (DAT), respectively. This in turn leads to increased extracellular concentrations of both norepinephrine and dopamine and, therefore, an increase in adrenergic and dopaminergic neurotransmission.

JZ-IV-10 is a piperidine derivative related to cocaine which acts as a highly potent serotonin–norepinephrine–dopamine reuptake inhibitor. The eugeroic modafinil was used as a lead to fuel this compound's discovery.
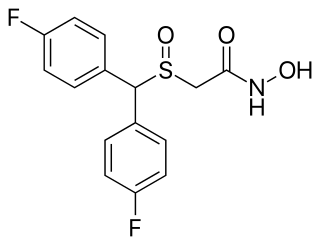
Fladrafinil, also known as fluorafinil or as bisfluoroadrafinil, is a wakefulness-promoting agent related to modafinil that was never marketed. It is sold online and used non-medically as a nootropic.
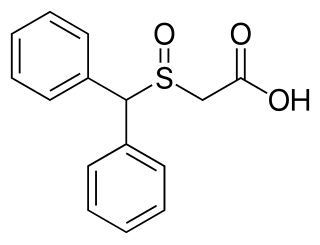
Modafinil acid (code name CRL-40467), also known as modafinilic acid or modafinil carboxylate, is one of the two major metabolites of modafinil – the other being modafinil sulfone. Modafinil acid is also a metabolite of the modafinil prodrug, adrafinil, and the (R)-(–)-enantiomer is a metabolite of armodafinil, the (R)-(–)-enantiomer of modafinil. Between 30 - 60% of modafinil is converted to modafinil acid and its half life is roughly half that of modafinil (about 7 hours). Modafinil acid seems to be inactive, and similarly to modafinil sulfone, does not appear to contribute to the wakefulness-promoting/psychostimulant effects of modafinil.

Modafinil sulfone (code name CRL-41056) is an achiral, oxidized metabolite of modafinil, a wakefulness-promoting agent. It is one of two major circulating metabolites of modafinil, the other being modafinil acid. Modafinil sulfone is also a metabolite of the modafinil prodrug, adrafinil. Modafinil sulfone is also a metabolite of armodafinil, the (R)-(–)-enantiomer of modafinil, as oxidation to the sulfone removes the chiral center at the sulfur atom. Modafinil sulfone has been described as inactive, and similarly to modafinil acid, does not appear to contribute to the wakefulness-promoting effects of modafinil. However, like modafinil, modafinil sulfone was found to show anticonvulsant properties in animals, indicating that it does possess some biological activity.

CE-123 is an analog of modafinil, the most researched of a series of structurally related heterocyclic derivatives. In animal studies, CE-123 was found to improve performance on tests of learning and memory in a manner consistent with a nootropic effect profile.
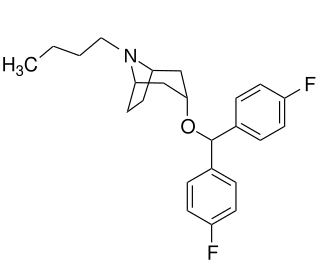
JHW-007 is a cocaine analog and a high affinity atypical dopamine reuptake inhibitor that is being researched for the treatment of cocaine addiction. JHW-007 has been found to blunt the psychostimulatory effects of cocaine and reduce self-administration in rodents. JHW-007 exposure has been shown to block the conditioned place preference effects of cocaine. JHW-007 may directly antagonize the autoregulatory dopamine D2 receptor, a hypothesis that was developed following the observation of JHW-007's ability to inhibit D2 receptor-mediated currents in the midbrain.

Esmodafinil is the enantiopure isolation of the (S) enantiomer of modafinil. Unlike armodafinil, esmodafinil has never been marketed on its own.

















