
The α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (also known as AMPA receptor, AMPAR, or quisqualate receptor) is an ionotropic transmembrane receptor for glutamate (iGluR) and predominantly Na+ ion channel that mediates fast synaptic transmission in the central nervous system (CNS). It has been traditionally classified as a non-NMDA-type receptor, along with the kainate receptor. Its name is derived from its ability to be activated by the artificial glutamate analog AMPA. The receptor was first named the "quisqualate receptor" by Watkins and colleagues after a naturally occurring agonist quisqualate and was only later given the label "AMPA receptor" after the selective agonist developed by Tage Honore and colleagues at the Royal Danish School of Pharmacy in Copenhagen. The GRIA2-encoded AMPA receptor ligand binding core (GluA2 LBD) was the first glutamate receptor ion channel domain to be crystallized.

Ligand-gated ion channels (LICs, LGIC), also commonly referred to as ionotropic receptors, are a group of transmembrane ion-channel proteins which open to allow ions such as Na+, K+, Ca2+, and/or Cl− to pass through the membrane in response to the binding of a chemical messenger (i.e. a ligand), such as a neurotransmitter.

CX717 is an ampakine compound created by Christopher Marrs and Gary Rogers in 1996 at Cortex Pharmaceuticals. It affects the neurotransmitter glutamate, with trials showing the drug improves cognitive functioning and memory.
Racetams are a class of drugs that share a pyrrolidone nucleus. Some, such as piracetam, aniracetam, oxiracetam, pramiracetam and phenylpiracetam are considered nootropics. Others such as levetiracetam, brivaracetam, and seletracetam are anticonvulsants.

Glutamate receptors are synaptic and non synaptic receptors located primarily on the membranes of neuronal and glial cells. Glutamate is abundant in the human body, but particularly in the nervous system and especially prominent in the human brain where it is the body's most prominent neurotransmitter, the brain's main excitatory neurotransmitter, and also the precursor for GABA, the brain's main inhibitory neurotransmitter. Glutamate receptors are responsible for the glutamate-mediated postsynaptic excitation of neural cells, and are important for neural communication, memory formation, learning, and regulation.
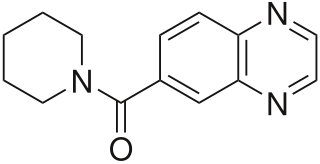
CX-516 is an ampakine and nootropic that acts as an AMPA receptor positive allosteric modulator and had been undergoing development by a collaboration between Cortex, Shire, and Servier. It was studied as a potential treatment for Alzheimer's disease under the brand name Ampalex, and was also being examined as a treatment for ADHD.

Farampator is an ampakine drug. It was developed by Cortex Pharmaceuticals, and licensed to Organon BioSciences for commercial development. Following the purchase of Organon by Schering-Plough in 2007, the development license to farampator was transferred. The development of farampator was eventually terminated, reportedly due to concerns about cardiac toxicity.
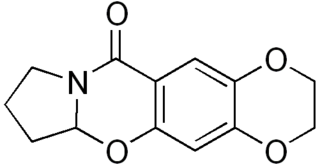
CX-614 is an ampakine drug developed by Cortex Pharmaceuticals. It has been investigated for its effect on AMPA receptors.

CX-546 is an ampakine drug developed by Cortex Pharmaceuticals.

Metabotropic glutamate receptor 2 (mGluR2) is a protein that, in humans, is encoded by the GRM2 gene. mGluR2 is a G protein-coupled receptor (GPCR) that couples with the Gi alpha subunit. The receptor functions as an autoreceptor for glutamate, that upon activation, inhibits the emptying of vesicular contents at the presynaptic terminal of glutamatergic neurons.
RespireRx Pharmaceuticals Inc. is a pharmaceutical company based in Glen Rock, New Jersey specializing in positive allosteric modulators of the AMPA receptor known as Ampakines.

LY-404187 is an AMPA receptor positive allosteric modulator which was developed by Eli Lilly and Company. It is a member of the biarylpropylsulfonamide class of AMPA receptor potentiators.
In pharmacology and biochemistry, allosteric modulators are a group of substances that bind to a receptor to change that receptor's response to stimuli. Some of them, like benzodiazepines or alcohol, function as psychoactive drugs. The site that an allosteric modulator binds to is not the same one to which an endogenous agonist of the receptor would bind. Modulators and agonists can both be called receptor ligands.
Within the science of molecular biology and cell biology, for human genetics, the GRIA2 gene is located on chromosome 4q32-q33. The gene product is the ionotropic AMPA glutamate receptor 2. The protein belongs to a family of ligand-activated glutamate receptors that are sensitive to alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionate (AMPA). Glutamate receptors function as the main excitatory neurotransmitter at many synapses in the central nervous system. L-glutamate, an excitatory neurotransmitter, binds to the Gria2 resulting in a conformational change. This leads to the opening of the channel converting the chemical signal to an electrical impulse. AMPA receptors (AMPAR) are composed of four subunits, designated as GluR1 (GRIA1), GluR2 (GRIA2), GluR3 (GRIA3), and GluR4(GRIA4) which combine to form tetramers. They are usually heterotrimeric but can be homodimeric. Each AMPAR has four sites to which an agonist can bind, one for each subunit.[5]

Pesampator is a positive allosteric modulator (PAM) of the AMPA receptor (AMPAR), an ionotropic glutamate receptor, which is under development by Pfizer for the treatment of cognitive symptoms in schizophrenia. It was also under development for the treatment of age-related sensorineural hearing loss, but development for this indication was terminated due to insufficient effectiveness. As of July 2018, pesampator is in phase II clinical trials for cognitive symptoms in schizophrenia.

Mibampator is a positive allosteric modulator (PAM) of the AMPA receptor (AMPAR), an ionotropic glutamate receptor, which was under development by Eli Lilly for the treatment of agitation/aggression in Alzheimer's disease but was never marketed. It reached phase II clinical trials prior to the discontinuation of its development.

Tulrampator is a positive allosteric modulator (PAM) of the AMPA receptor (AMPAR), an ionotropic glutamate receptor, which is under development by RespireRx Pharmaceuticals and Servier for the treatment of major depressive disorder, Alzheimer's disease, dementia, and mild cognitive impairment. Tulrampator was in phase II clinical trial for depression, but failed to show superiority over placebo. There are also phase II clinical trials for Alzheimer's disease and phase I trials for dementia and mild cognitive impairment.

AMPA receptor positive allosteric modulators are positive allosteric modulators (PAMs) of the AMPA receptor (AMPR), a type of ionotropic glutamate receptor which mediates most fast synaptic neurotransmission in the central nervous system.

Willardiine (correctly spelled with two successive i's) or (S)-1-(2-amino-2-carboxyethyl)pyrimidine-2,4-dione is a chemical compound that occurs naturally in the seeds of Mariosousa willardiana and Acacia sensu lato. The seedlings of these plants contain enzymes capable of complex chemical substitutions that result in the formation of free amino acids (See:#Synthesis). Willardiine is frequently studied for its function in higher level plants. Additionally, many derivates of willardiine are researched for their potential in pharmaceutical development. Willardiine was first discovered in 1959 by R. Gmelin, when he isolated several free, non-protein amino acids from Acacia willardiana (another name for Mariosousa willardiana) when he was studying how these families of plants synthesize uracilyalanines. A related compound, Isowillardiine, was concurrently isolated by a different group, and it was discovered that the two compounds had different structural and functional properties. Subsequent research on willardiine has focused on the functional significance of different substitutions at the nitrogen group and the development of analogs of willardiine with different pharmacokinetic properties. In general, Willardiine is the one of the first compounds studied in which slight changes to molecular structure result in compounds with significantly different pharmacokinetic properties.















