
Gastrulation is the stage in the early embryonic development of most animals, during which the blastula, or in mammals the blastocyst, is reorganized into a two-layered or three-layered embryo known as the gastrula. Before gastrulation, the embryo is a continuous epithelial sheet of cells; by the end of gastrulation, the embryo has begun differentiation to establish distinct cell lineages, set up the basic axes of the body, and internalized one or more cell types including the prospective gut.
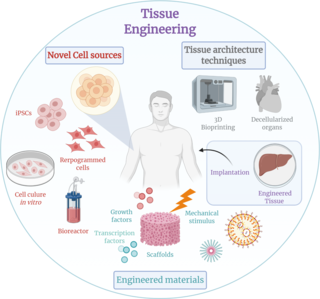
Tissue engineering is a biomedical engineering discipline that uses a combination of cells, engineering, materials methods, and suitable biochemical and physicochemical factors to restore, maintain, improve, or replace different types of biological tissues. Tissue engineering often involves the use of cells placed on tissue scaffolds in the formation of new viable tissue for a medical purpose, but is not limited to applications involving cells and tissue scaffolds. While it was once categorized as a sub-field of biomaterials, having grown in scope and importance, it can be considered as a field of its own.
Tissue culture is the growth of tissues or cells in an artificial medium separate from the parent organism. This technique is also called micropropagation. This is typically facilitated via use of a liquid, semi-solid, or solid growth medium, such as broth or agar. Tissue culture commonly refers to the culture of animal cells and tissues, with the more specific term plant tissue culture being used for plants. The term "tissue culture" was coined by American pathologist Montrose Thomas Burrows. This is possible only in certain conditions. It also requires more attention. It can be done only in genetic labs with various chemicals.

Plant callus is a growing mass of unorganized plant parenchyma cells. In living plants, callus cells are those cells that cover a plant wound. In biological research and biotechnology callus formation is induced from plant tissue samples (explants) after surface sterilization and plating onto tissue culture medium in vitro. The culture medium is supplemented with plant growth regulators, such as auxin, cytokinin, and gibberellin, to initiate callus formation or somatic embryogenesis. Callus initiation has been described for all major groups of land plants.

Embryonic stem cells (ESCs) are pluripotent stem cells derived from the inner cell mass of a blastocyst, an early-stage pre-implantation embryo. Human embryos reach the blastocyst stage 4–5 days post fertilization, at which time they consist of 50–150 cells. Isolating the inner cell mass (embryoblast) using immunosurgery results in destruction of the blastocyst, a process which raises ethical issues, including whether or not embryos at the pre-implantation stage have the same moral considerations as embryos in the post-implantation stage of development.
Cell culture or tissue culture is the process by which cells are grown under controlled conditions, generally outside of their natural environment. After cells of interest have been isolated from living tissue, they can subsequently be maintained under carefully controlled conditions. They need to be kept at body temperature (37 °C) in an incubator. These conditions vary for each cell type, but generally consist of a suitable vessel with a substrate or rich medium that supplies the essential nutrients (amino acids, carbohydrates, vitamins, minerals), growth factors, hormones, and gases (CO2, O2), and regulates the physio-chemical environment (pH buffer, osmotic pressure, temperature). Most cells require a surface or an artificial substrate to form an adherent culture as a monolayer (one single-cell thick), whereas others can be grown free floating in a medium as a suspension culture. This is typically facilitated via use of a liquid, semi-solid, or solid growth medium, such as broth or agar. Tissue culture commonly refers to the culture of animal cells and tissues, with the more specific term plant tissue culture being used for plants. The lifespan of most cells is genetically determined, but some cell-culturing cells have been 'transformed' into immortal cells which will reproduce indefinitely if the optimal conditions are provided.

Embryoid bodies (EBs) are three-dimensional aggregates formed by pluripotent stem cells. These include embryonic stem cells (ESC) and induced pluripotent stem cells (iPSC)
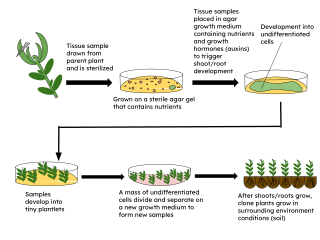
Micropropagation or tissue culture is the practice of rapidly multiplying plant stock material to produce many progeny plants, using modern plant tissue culture methods.

A stem cell line is a group of stem cells that is cultured in vitro and can be propagated indefinitely. Stem cell lines are derived from either animal or human tissues and come from one of three sources: embryonic stem cells, adult stem cells, or induced pluripotent stem cells. They are commonly used in research and regenerative medicine.

The stem cell controversy concerns the ethics of research involving the development and use of human embryos. Most commonly, this controversy focuses on embryonic stem cells. Not all stem cell research involves human embryos. For example, adult stem cells, amniotic stem cells, and induced pluripotent stem cells do not involve creating, using, or destroying human embryos, and thus are minimally, if at all, controversial. Many less controversial sources of acquiring stem cells include using cells from the umbilical cord, breast milk, and bone marrow, which are not pluripotent.
In biology, explant culture is a technique to organotypically culture cells from a piece or pieces of tissue or organ removed from a plant or animal. The term explant can be applied to samples obtained from any part of the organism. The extraction process is extensively sterilized, and the culture can be typically used for two to three weeks.

Fate mapping is a method used in developmental biology to study the embryonic origin of various adult tissues and structures. The "fate" of each cell or group of cells is mapped onto the embryo, showing which parts of the embryo will develop into which tissue. When carried out at single-cell resolution, this process is called cell lineage tracing. It is also used to trace the development of tumors. Fate mapping and cell lineage are similar methods for tracing the history of cells.
Embryomics is the identification, characterization and study of the diverse cell types which arise during embryogenesis, especially as this relates to the location and developmental history of cells in the embryo. Cell type may be determined according to several criteria: location in the developing embryo, gene expression as indicated by protein and nucleic acid markers and surface antigens, and also position on the embryogenic tree.

Plant tissue culture is a collection of techniques used to maintain or grow plant cells, tissues, or organs under sterile conditions on a nutrient culture medium of known composition. It is widely used to produce clones of a plant in a method known as micropropagation. Different techniques in plant tissue culture may offer certain advantages over traditional methods of propagation, including:
Embryo rescue is one of the earliest and successful forms of in-vitro culture techniques that is used to assist in the development of plant embryos that might not survive to become viable plants. Embryo rescue plays an important role in modern plant breeding, allowing the development of many interspecific and intergeneric food and ornamental plant crop hybrids. This technique nurtures the immature or weak embryo, thus allowing it the chance to survive. Plant embryos are multicellular structures that have the potential to develop into a new plant. The most widely used embryo rescue procedure is referred to as embryo culture, and involves excising plant embryos and placing them onto media culture. Embryo rescue is most often used to create interspecific and intergeneric crosses that would normally produce seeds which are aborted. Interspecific incompatibility in plants can occur for many reasons, but most often embryo abortion occurs In plant breeding, wide hybridization crosses can result in small shrunken seeds which indicate that fertilization has occurred, however the seed fails to develop. Many times, remote hybridizations will fail to undergo normal sexual reproduction, thus embryo rescue can assist in circumventing this problem.
Secretomics is a type of proteomics which involves the analysis of the secretome—all the secreted proteins of a cell, tissue or organism. Secreted proteins are involved in a variety of physiological processes, including cell signaling and matrix remodeling, but are also integral to invasion and metastasis of malignant cells. Secretomics has thus been especially important in the discovery of biomarkers for cancer and understanding molecular basis of pathogenesis. The analysis of the insoluble fraction of the secretome has been termed matrisomics.

Montrose Thomas Burrows was a US surgeon and pathologist specializing in cancer research and surgery. He was born into a Scots-Irish Presbyterian family in Halstead, Kansas.

Margaret Adaline Reed Lewis (1881–1970) was an American cell biologist and embryologist who made contributions to cancer research and cell culture techniques, and was likely the first person to successfully grow mammalian tissue in vitro. She authored around 150 papers, many co-authored with her husband Warren Harmon Lewis. The Lewises developed a growth medium called the Locke-Lewis solution and jointly received the Gerhard Gold Medal from the Pathological Society of Philadelphia.
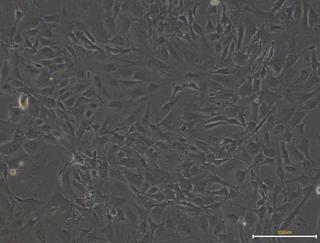
Mouse Embryonic Fibroblasts (MEFs) are a type of fibroblast prepared from mouse embryo. MEFs show a spindle shape when cultured in vitro, a typical feature of fibroblasts. The MEF is a limited cell line. After several transmissions, MEFs will senesce and finally die off. Nevertheless, researchers can use several strategies, like virus infection or repeated transmission to immortalize MEF cells, which can let MEFs grown indefinitely in spite of some changes in characters.
Ovarian culture is an in-vitro process that allows for the investigation of the development, toxicology and pathology of the ovary. This technique can also be used to study possible applications of fertility treatments e.g. isolating oocytes from primordial ovarian follicles that could be used for fertilisation.














