Charcot–Marie–Tooth disease (CMT) is a hereditary motor and sensory neuropathy of the peripheral nervous system characterized by progressive loss of muscle tissue and touch sensation across various parts of the body. This disease is the most commonly inherited neurological disorder, affecting about one in 2,500 people. It is named after those who classically described it: the Frenchman Jean-Martin Charcot (1825–1893), his pupil Pierre Marie (1853–1940), and the Briton Howard Henry Tooth (1856–1925).

Fragile X syndrome (FXS) is a genetic neurodevelopmental disorder characterized by mild-to-moderate intellectual disability. The average IQ in males with FXS is under 55, while about two thirds of affected females are intellectually disabled. Physical features may include a long and narrow face, large ears, flexible fingers, and large testicles. About a third of those affected have features of autism such as problems with social interactions and delayed speech. Hyperactivity is common, and seizures occur in about 10%. Males are usually more affected than females.

Lissencephaly is a set of rare brain disorders whereby the whole or parts of the surface of the brain appear smooth. It is caused by defective neuronal migration during the 12th to 24th weeks of gestation, resulting in a lack of development of brain folds (gyri) and grooves (sulci). It is a form of cephalic disorder. Terms such as agyria and pachygyria are used to describe the appearance of the surface of the brain.

Occipital horn syndrome (OHS), formerly considered a variant of Ehlers–Danlos syndrome, is an X-linked recessive mitochondrial and connective tissue disorder. It is caused by a deficiency in the transport of the essential mineral copper, associated with mutations in the ATP7A gene.

The heritability of autism is the proportion of differences in expression of autism that can be explained by genetic variation; if the heritability of a condition is high, then the condition is considered to be primarily genetic. Autism has a strong genetic basis. Although the genetics of autism are complex, autism spectrum disorder (ASD) is explained more by multigene effects than by rare mutations with large effects.

MECP2 is a gene that encodes the protein MECP2. MECP2 appears to be essential for the normal function of nerve cells. The protein seems to be particularly important for mature nerve cells, where it is present in high levels. The MECP2 protein is likely to be involved in turning off several other genes. This prevents the genes from making proteins when they are not needed. Recent work has shown that MECP2 can also activate other genes. The MECP2 gene is located on the long (q) arm of the X chromosome in band 28 ("Xq28"), from base pair 152,808,110 to base pair 152,878,611.

Many causes of autism, including environmental and genetic factors, have been recognized or proposed, but understanding of the theory of causation of autism is incomplete. Attempts have been made to incorporate the known genetic and environmental causes into a comprehensive causative framework. ASD is a neurodevelopmental disorder marked by impairments in communicative ability and social interaction, as well as restricted and repetitive behaviors, interests, or activities not suitable for the individual's developmental stage. The severity of symptoms and functional impairment vary between individuals.
In genetics and cell biology, repression is a mechanism often used to decrease or inhibit the expression of a gene. Removal of repression is called derepression. This mechanism may occur at different stages in the expression of a gene, with the result of increasing the overall RNA or protein products. Dysregulation of derepression mechanisms can result in altered gene expression patterns, which may lead to negative phenotypic consequences such as disease.

CDKL5 is a gene that provides instructions for making a protein called cyclin-dependent kinase-like 5 also known as serine/threonine kinase 9 (STK9) that is essential for normal brain development. Mutations in the gene can cause deficiencies in the protein. The gene regulates neuronal morphology through cytoplasmic signaling and controlling gene expression. The CDKL5 protein acts as a kinase, which is an enzyme that changes the activity of other proteins by adding a cluster of oxygen and phosphorus atoms at specific positions. Researchers are currently working to determine which proteins are targeted by the CDKL5 protein.

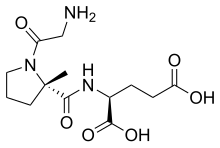
Gamma-aminobutyric acid receptor subunit beta-3 is a protein that in humans is encoded by the GABRB3 gene. It is located within the 15q12 region in the human genome and spans 250kb. This gene includes 10 exons within its coding region. Due to alternative splicing, the gene codes for many protein isoforms, all being subunits in the GABAA receptor, a ligand-gated ion channel. The beta-3 subunit is expressed at different levels within the cerebral cortex, hippocampus, cerebellum, thalamus, olivary body and piriform cortex of the brain at different points of development and maturity. GABRB3 deficiencies are implicated in many human neurodevelopmental disorders and syndromes such as Angelman syndrome, Prader-Willi syndrome, nonsyndromic orofacial clefts, epilepsy and autism. The effects of methaqualone and etomidate are mediated through GABBR3 positive allosteric modulation.
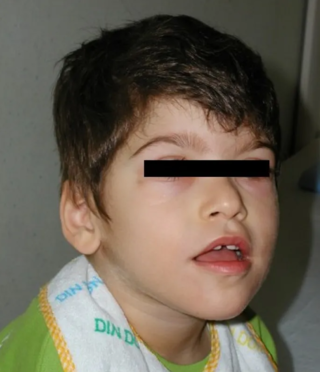
Pitt–Hopkins syndrome (PTHS) is a rare genetic disorder characterized by developmental delay, moderate to severe intellectual disability, distinctive facial features, and possible intermittent hyperventilation followed by apnea. Epilepsy often occurs in Pitt-Hopkins. It is part of the clinical spectrum of Rett-like syndromes. Pitt-Hopkins syndrome is clinically similar to Angelman syndrome, Rett-syndrome, Mowat Wilson syndrome, and ATR-X syndrome.

Huda Yahya Zoghbi is a Lebanese-born American geneticist, and a professor at the Departments of Molecular and Human Genetics, Neuroscience and Neurology at the Baylor College of Medicine. She is the director of the Jan and Dan Duncan Neurological Research Institute. She was the editor of the Annual Review of Neuroscience from 2018-2024.
Michele Zappella is an Italian psychiatrist and scholar of Child Neuropsychiatry. He is a native of Viareggio, Italy.
Epilepsy-intellectual disability in females also known as PCDH19 gene-related epilepsy or epileptic encephalopathy, early infantile, 9 (EIEE9), is a rare type of epilepsy that affects predominantly females and is characterized by clusters of brief seizures, which start in infancy or early childhood, and is occasionally accompanied by varying degrees of cognitive impairment. The striking pattern of onset seizures at a young age, genetic testing and laboratory results, potential developmental delays or developmental regression and associated disorders, eases diagnosis.
Autism spectrum disorder (ASD) refers to a variety of conditions typically identified by challenges with social skills, communication, speech, and repetitive sensory-motor behaviors. The 11th International Classification of Diseases (ICD-11), released in January 2021, characterizes ASD by the associated deficits in the ability to initiate and sustain two-way social communication and restricted or repetitive behavior unusual for the individual's age or situation. Although linked with early childhood, the symptoms can appear later as well. Symptoms can be detected before the age of two and experienced practitioners can give a reliable diagnosis by that age. However, official diagnosis may not occur until much older, even well into adulthood. There is a large degree of variation in how much support a person with ASD needs in day-to-day life. This can be classified by a further diagnosis of ASD level 1, level 2, or level 3. Of these, ASD level 3 describes people requiring very substantial support and who experience more severe symptoms. ASD-related deficits in nonverbal and verbal social skills can result in impediments in personal, family, social, educational, and occupational situations. This disorder tends to have a strong correlation with genetics along with other factors. More research is identifying ways in which epigenetics is linked to autism. Epigenetics generally refers to the ways in which chromatin structure is altered to affect gene expression. Mechanisms such as cytosine regulation and post-translational modifications of histones. Of the 215 genes contributing, to some extent in ASD, 42 have been found to be involved in epigenetic modification of gene expression. Some examples of ASD signs are specific or repeated behaviors, enhanced sensitivity to materials, being upset by changes in routine, appearing to show reduced interest in others, avoiding eye contact and limitations in social situations, as well as verbal communication. When social interaction becomes more important, some whose condition might have been overlooked suffer social and other exclusion and are more likely to have coexisting mental and physical conditions. Long-term problems include difficulties in daily living such as managing schedules, hypersensitivities, initiating and sustaining relationships, and maintaining jobs.

MECP2 duplication syndrome (M2DS) is a rare disease that is characterized by severe intellectual disability and impaired motor function. It is an X-linked genetic disorder caused by the overexpression of MeCP2 protein.

L1 syndrome is a group of mild to severe X-linked recessive disorders that share a common genetic basis. The spectrum of L1 syndrome disorders includes X-linked complicated corpus callosum dysgenesis, spastic paraplegia 1, MASA syndrome, and X-linked hydrocephalus with stenosis of the aqueduct of Sylvius (HSAS). It is also called L1CAM syndrome and CRASH syndrome, an acronym for its primary clinical features: corpus callosum hypoplasia, retardation, adducted thumbs, spasticity, and hydrocephalus.
There is no cure for Rett syndrome. Treatment is directed towards improving function and addressing symptoms throughout life. A multi-disciplinary team approach is typically used to treat the person throughout life. This team may include primary care physician, physical therapist, occupational therapist, speech-language pathologist, nutritionist, and support services in academic and occupational settings.

SRD5A3-CDG is a rare, non X-linked congenital disorder of glycosylation (CDG) due to a mutation in the steroid 5 alpha reductase type 3 gene. It is one of over 150 documented types of Congenital disorders of Glycosylation. Like many other CDGs, SRD5A3 is ultra-rare, with around 38 documented cases in the world.
CDKL5 deficiency disorder (CDD) is a rare genetic disorder caused by pathogenic variants in the gene CDKL5.