
Atropine is a tropane alkaloid and anticholinergic medication used to treat certain types of nerve agent and pesticide poisonings as well as some types of slow heart rate, and to decrease saliva production during surgery. It is typically given intravenously or by injection into a muscle. Eye drops are also available which are used to treat uveitis and early amblyopia. The intravenous solution usually begins working within a minute and lasts half an hour to an hour. Large doses may be required to treat some poisonings.

Xylometazoline, also spelled xylomethazoline, is a drug used to reduce symptoms of nasal congestion, allergic rhinitis, and sinusitis. Use is not recommended for more than seven days. Use is also not recommended in those less than three months of age and some say not less than 6 years of age. It is used directly in the nose as a spray or drops.
An International Nonproprietary Name (INN) is an official generic and nonproprietary name given to a pharmaceutical substance or an active ingredient. INNs are intended to make communication more precise by providing a unique standard name for each active ingredient, to avoid prescribing errors. The INN system was initiated by the World Health Organization (WHO) in 1953.

Dimethoxybromoamphetamine (DOB), also known as brolamfetamine and bromo-DMA, is a psychedelic drug and substituted amphetamine of the phenethylamine class of compounds. DOB was first synthesized by Alexander Shulgin in 1967. Its synthesis and effects are documented in Shulgin's book PiHKAL: A Chemical Love Story.

3,4-Methylenedioxyamphetamine (MDA), sometimes referred to as “sass,” is an empathogen-entactogen, stimulant, and psychedelic drug of the amphetamine family that is encountered mainly as a recreational drug. In its pharmacology, MDA is a serotonin–norepinephrine–dopamine releasing agent (SNDRA). In most countries, the drug is a controlled substance and its possession and sale are illegal.
Thailand's Psychotropic Substances Act is a law designed to regulate certain mind-altering drugs. According to the Office of the Narcotics Control Board, "The Act directly resulted from the Convention on Psychotropic Substances 1971 of which Thailand is a party." The Act divides psychotropic drugs into four Schedules. Offenses involving Schedule I and II drugs carry heavier penalties than those involving Schedule III and IV drugs. Note that this statute does not regulate most opioids, cocaine, or some amphetamines. The vast majority of narcotic painkillers, along with cocaine and most amphetamines are regulated under the Narcotics Act.
The Controlled Drugs and Substances Act is Canada's federal drug control statute. Passed in 1996 under Prime Minister Jean Chrétien's government, it repeals the Narcotic Control Act and Parts III and IV of the Food and Drugs Act, and establishes eight Schedules of controlled substances and two Classes of precursors. It provides that "The Governor in Council may, by order, amend any of Schedules I to VIII by adding to them or deleting from them any item or portion of an item, where the Governor in Council deems the amendment to be necessary in the public interest."

Cefepime is a fourth-generation cephalosporin antibiotic. Cefepime has an extended spectrum of activity against Gram-positive and Gram-negative bacteria, with greater activity against both types of organism than third-generation agents. A 2007 meta-analysis suggested when data of trials were combined, mortality was increased in people treated with cefepime compared with other β-lactam antibiotics. In response, the U.S. Food and Drug Administration (FDA) performed their own meta-analysis which found no mortality difference.

Methylergometrine, also known as methylergonovine and sold under the brand name Methergine, is a medication of the ergoline and lysergamide groups which is used as an oxytocic in obstetrics and as an antimigraine agent in the treatment of migraine headaches. It reportedly produces psychedelic effects similar to those of lysergic acid diethylamide (LSD) at high doses.

Dextromoramide is a powerful opioid analgesic approximately three times more potent than morphine but shorter acting. It is subject to drug prohibition regimes, both internationally through UN treaties and by the criminal law of individual nations, and is usually prescribed only in the Netherlands.
Chloroxylenol, also known as para-chloro-meta-xylenol (PCMX), is a chlorine substituted phenol with a white to off-white appearance and a phenolic odor.
The nomenclature of monoclonal antibodies is a naming scheme for assigning generic, or nonproprietary, names to monoclonal antibodies. An antibody is a protein that is produced in B cells and used by the immune system of humans and other vertebrate animals to identify a specific foreign object like a bacterium or a virus. Monoclonal antibodies are those that were produced in identical cells, often artificially, and so share the same target object. They have a wide range of applications including medical uses.

Nemonapride, also previously known as emonapride and sold under the brand name Emilace, is an atypical antipsychotic which is used in the treatment of schizophrenia. It is taken by mouth.
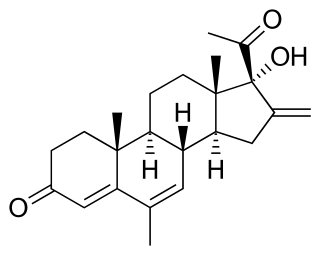
Melengestrol is a steroidal progestin of the 17α-hydroxyprogesterone group and an antineoplastic drug which was never marketed. An acylated derivative, melengestrol acetate, is used as a growth promoter in animals.

Lomevactone is a drug described as a psychostimulant and antidepressant which was synthesized and assayed in the 1980s, but was never marketed.

Morforex, also referable to as N-morpholinoethylamphetamine, is an anorectic which was never marketed.
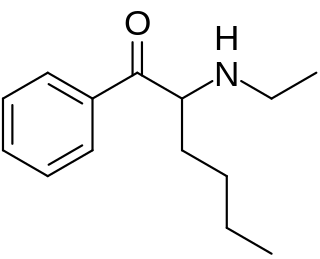
N-Ethylhexedrone (also known as α-ethylaminocaprophenone, N-ethylnorhexedrone, hexen, and NEH) is a stimulant of the cathinone class that acts as a norepinephrine–dopamine reuptake inhibitor (NDRI) with IC50 values of 0.0978 and 0.0467 μM, respectively. N-Ethylhexedrone was first mentioned in a series of patents by Boehringer Ingelheim in the 1960s which led to the development of the better-known drug methylenedioxypyrovalerone (MDPV). Since the mid-2010s, N-ethylhexedrone has been sold online as a designer drug. In 2018, N-ethylhexedrone was the second most common drug of the cathinone class to be identified in Drug Enforcement Administration seizures.
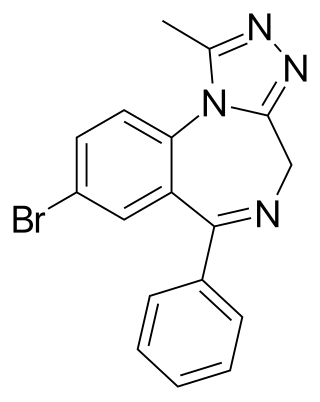
Bromazolam (XLI-268) is a triazolobenzodiazepine (TBZD) which was first synthesised in 1976, but was never marketed. It has subsequently been sold as a designer drug, first being definitively identified by the EMCDDA in Sweden in 2016. It is the bromo instead of chloro analogue of alprazolam and has similar sedative and anxiolytic effects to it and other benzodiazepines. Bromazolam is a non subtype selective agonist at the benzodiazepine site of GABAA receptors, with a binding affinity of 2.81 nM at the α1 subtype, 0.69 nM at α2 and 0.62 nM at α5. The "common" dosage range for users of bromazolam was reported to be 1–2 mg, suggesting its potency is similar to alprazolam.













