
Amphetamine is a central nervous system (CNS) stimulant that is used in the treatment of attention deficit hyperactivity disorder (ADHD), narcolepsy, and obesity. Amphetamine was discovered as a chemical in 1887 by Lazăr Edeleanu, and then as a drug in the late 1920s. It exists as two enantiomers: levoamphetamine and dextroamphetamine. Amphetamine properly refers to a specific chemical, the racemic free base, which is equal parts of the two enantiomers in their pure amine forms. The term is frequently used informally to refer to any combination of the enantiomers, or to either of them alone. Historically, it has been used to treat nasal congestion and depression. Amphetamine is also used as an athletic performance enhancer and cognitive enhancer, and recreationally as an aphrodisiac and euphoriant. It is a prescription drug in many countries, and unauthorized possession and distribution of amphetamine are often tightly controlled due to the significant health risks associated with recreational use.
An anxiolytic is a medication or other intervention that reduces anxiety. This effect is in contrast to anxiogenic agents which increase anxiety. Anxiolytic medications are used for the treatment of anxiety disorders and their related psychological and physical symptoms.

Stimulants are a class of drugs that increase the activity of the brain. They are used for various purposes, such as enhancing alertness, attention, motivation, cognition, mood, and physical performance. Some of the most common stimulants are caffeine, nicotine, amphetamines, cocaine, methylphenidate, and modafinil.

Methylphenidate, sold under the brand names Ritalin and Concerta among others, is a central nervous system (CNS) stimulant used medically to treat attention deficit hyperactivity disorder (ADHD) and, to a lesser extent, narcolepsy. It is a primary medication for ADHD ; it may be taken by mouth or applied to the skin, and different formulations have varying durations of effect. For ADHD, the effectiveness of methylphenidate is comparable to atomoxetine but modestly lower than amphetamines, alleviating the executive functioning deficits of sustained attention, inhibition, working memory, reaction time and emotional self-regulation.
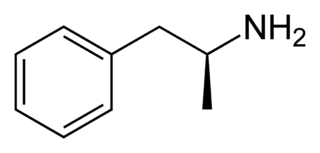
Dextroamphetamine (INN:dexamfetamine) is a potent central nervous system (CNS) stimulant and enantiomer of amphetamine that is prescribed for the treatment of attention deficit hyperactivity disorder (ADHD) and narcolepsy. It is also used as an athletic performance and cognitive enhancer, and recreationally as an aphrodisiac and euphoriant. Dextroamphetamine is generally regarded as the prototypical stimulant.
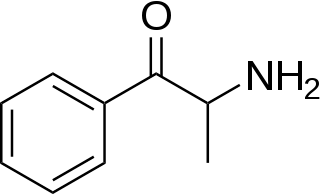
Cathinone is a monoamine alkaloid found in the shrub Catha edulis (khat) and is chemically similar to ephedrine, cathine, methcathinone and other amphetamines. It is probably the main contributor to the stimulant effect of Catha edulis, also known as khat. Cathinone differs from many other amphetamines in that it has a ketone functional group. Other phenethylamines that share this structure include the stimulants methcathinone, MDPV, mephedrone and the antidepressant bupropion.
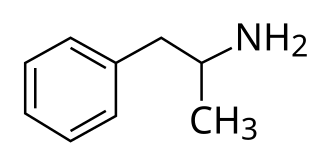
Adderall and Mydayis are trade names for a combination drug containing four salts of amphetamine. The mixture is composed of equal parts racemic amphetamine and dextroamphetamine, which produces a (3:1) ratio between dextroamphetamine and levoamphetamine, the two enantiomers of amphetamine. Both enantiomers are stimulants, but differ enough to give Adderall an effects profile distinct from those of racemic amphetamine or dextroamphetamine, which are marketed as Evekeo and Dexedrine/Zenzedi, respectively. Adderall is used in the treatment of attention deficit hyperactivity disorder (ADHD) and narcolepsy. It is also used illicitly as an athletic performance enhancer, cognitive enhancer, appetite suppressant, and recreationally as a euphoriant. It is a central nervous system (CNS) stimulant of the phenethylamine class.

Nootropics, colloquially brain supplements, smart drugs and cognitive enhancers, are natural, semisynthetic or synthetic compounds which purportedly improve cognitive functions, such as executive functions, attention or memory.

Phenylpiracetam, also known as fonturacetam and sold under the brand names Phenotropil, Actitropil, and Carphedon among others, is a stimulant and nootropic medication used in Russia and certain other Eastern European countries in the treatment of cerebrovascular deficiency, depression, apathy, and attention, and memory problems, among other indications. It is also used in Russian cosmonauts to improve physical, mental, and cognitive abilities. The drug is taken by mouth.

Phenylacetone, also known as phenyl-2-propanone, is an organic compound with the chemical formula C6H5CH2COCH3. It is a colorless oil that is soluble in organic solvents. It is a mono-substituted benzene derivative, consisting of an acetone attached to a phenyl group. As such, its systematic IUPAC name is 1-phenyl-2-propanone.

Arecoline is a nicotinic acid-based mild parasympathomimetic stimulant alkaloid found in the areca nut, the fruit of the areca palm. It is an odourless oily liquid. It can bring a sense of enhanced alertness and energy along with mild feelings of euphoria and relaxation.

Picamilon is a drug formed by a synthetic combination of niacin and γ-aminobutyric acid (GABA). It was developed in the Soviet Union in 1969 and further studied in both Russia and Japan as a prodrug of GABA.
Performance-enhancing substances, also known as performance-enhancing drugs (PEDs), are substances that are used to improve any form of activity performance in humans.

Troparil is a stimulant drug used in scientific research. Troparil is a phenyltropane-based dopamine reuptake inhibitor (DRI) that is derived from methylecgonidine. Troparil is a few times more potent than cocaine as a dopamine reuptake inhibitor, but is less potent as a serotonin reuptake inhibitor, and has a duration spanning a few times longer, since the phenyl ring is directly connected to the tropane ring through a non-hydrolyzable carbon-carbon bond. The lack of an ester linkage removes the local anesthetic action from the drug, so troparil is a pure stimulant. This change in activity also makes troparil slightly less cardiotoxic than cocaine. The most commonly used form of troparil is the tartrate salt, but the hydrochloride and naphthalenedisulfonate salts are also available, as well as the free base.
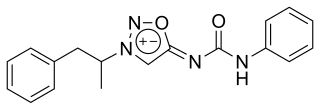
Mesocarb, sold under the brand name Sidnocarb or Sydnocarb and known by the developmental code name MLR-1017, is a psychostimulant medication which has been used in the treatment of psychiatric disorders and for a number of other indications in the Soviet Union and Russia. It is currently under development for the treatment of Parkinson's disease and sleep disorders. It is taken by mouth.
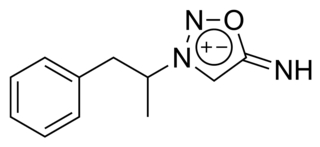
Feprosidnine (Sydnophen) is a stimulant drug which was developed in the USSR in the 1970s. It is structurally related to another Russian drug mesocarb but unlike mesocarb, was withdrawn earlier from production. In comparison with mesocarb it has own antidepressant activity, which makes it useful in treating depressions. Indications of feprosidnine included apathic, asthenic depressions, fatigue, apathic syndrome, narcolepsy and other similar conditions. Therapeutic range of doses: 10-50mg a day. Sydnophen has multiple mechanisms of action, the relative importance of which has not been clearly established. Effects on the body include reversible monoamine oxidase inhibition, cholinergic, adrenergic, opioid and nitric oxide donating actions, all of which may contribute to its pharmacological effects to some extent.

Bromantane, sold under the brand name Ladasten, is an atypical central nervous system (CNS) stimulant and anxiolytic drug of the adamantane family that is related to amantadine and memantine. Medically, it is approved in Russia for the treatment of neurasthenia. Although the effects of bromantane have been determined to be dependent on the dopaminergic and possibly serotonergic neurotransmitter systems, its exact mechanism of action is unknown, and is distinct in its properties relative to typical stimulants such as amphetamine. Bromantane has sometimes been described as an actoprotector.
Substituted amphetamines are a class of compounds based upon the amphetamine structure; it includes all derivative compounds which are formed by replacing, or substituting, one or more hydrogen atoms in the amphetamine core structure with substituents. The compounds in this class span a variety of pharmacological subclasses, including stimulants, empathogens, and hallucinogens, among others. Examples of substituted amphetamines are amphetamine (itself), methamphetamine, ephedrine, cathinone, phentermine, mephentermine, tranylcypromine, bupropion, methoxyphenamine, selegiline, amfepramone (diethylpropion), pyrovalerone, MDMA (ecstasy), and DOM (STP).

G-130 is a drug with stimulant and anorectic effects, related to phenmetrazine.

Pyridoxiphen, also known as amphetamine–pyridoxine condensation product or as pyridoxylamphetamine, is a drug of the substituted amphetamine family which was developed in the Soviet Union in the 1960s. It is the condensation product of amphetamine (phenamine) and pyridoxine (vitamin B6). It was developed for potential treatment of central nervous system conditions. The drug has sympatholytic and hypotensive effects in animals. It is highly ionic and may not be able to cross the blood–brain barrier.

















