The Misuse of Drugs Act 1971 is an act of the Parliament of the United Kingdom. It represents action in line with treaty commitments under the Single Convention on Narcotic Drugs, the Convention on Psychotropic Substances, and the United Nations Convention Against Illicit Traffic in Narcotic Drugs and Psychotropic Substances.
A designer drug is a structural or functional analog of a controlled substance that has been designed to mimic the pharmacological effects of the original drug, while avoiding classification as illegal and/or detection in standard drug tests. Designer drugs include psychoactive substances that have been designated by the European Union, Australia, and New Zealand, as new psychoactive substances (NPS) as well as analogs of performance-enhancing drugs such as designer steroids.
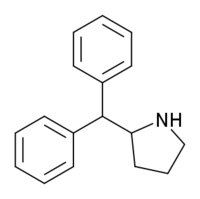
AL-LAD, also known as 6-allyl-6-nor-LSD, is a psychedelic drug and an analog of lysergic acid diethylamide (LSD). It is described by Alexander Shulgin in the book TiHKAL. It is synthesized starting from nor-LSD as a precursor, using allyl bromide as a reactant.

PRO-LAD is an analogue of LSD. It is described by Alexander Shulgin in the book TiHKAL. PRO-LAD is a psychedelic drug similar to LSD, and is around as potent as LSD itself with an active dose reported at between 100 and 200 micrograms.

Phenazepam is a benzodiazepine drug, first developed in the Soviet Union in 1975, and now produced in Russia and several other countries.

Methylenedioxypyrovalerone is a stimulant of the cathinone class that acts as a norepinephrine–dopamine reuptake inhibitor (NDRI). It was first developed in the 1960s by a team at Boehringer Ingelheim. Its activity at the dopamine transporter is six times stronger than at the norepinephrine transporter and it is virtually inactive at the serotonin transporter. MDPV remained an obscure stimulant until around 2004 when it was reportedly sold as a designer drug. In the US, products containing MDPV and labeled as bath salts were sold as recreational drugs in gas stations, similar to the marketing for Spice and K2 as incense, until it was banned in 2011.

Pipradrol, also known by its brand name Meratran, is a mild central nervous system stimulant that acts as a norepinephrine-dopamine reuptake inhibitor. Developed in the United States in the 1940s and patented in 1953, pipradrol was initially marketed as an antidepressant in the mid-1950s. It was subsequently used as an adjunct treatment for various conditions, including obesity, senile dementia, narcolepsy, and schizophrenia.

Desoxypipradrol, also known as 2-diphenylmethylpiperidine (2-DPMP), is a drug developed by Ciba in the 1950s which acts as a norepinephrine-dopamine reuptake inhibitor (NDRI).
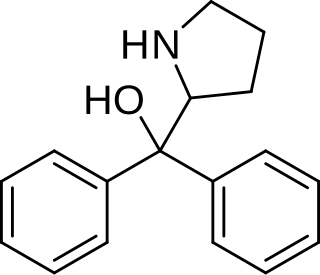
Diphenylprolinol (D2PM), or (R/S)-(±)-diphenyl-2-pyrrolidinyl-methanol, is a norepinephrine-dopamine reuptake inhibitor which is used as a designer drug.

Mephedrone, also known as 4-methylmethcathinone, 4-MMC, and 4-methylephedrone, is a synthetic stimulant drug belonging to the amphetamine and cathinone classes. It is commonly referred to by slang names such as drone, M-CAT, White Magic, meow meow,and bubble. Chemically, it is similar to the cathinone compounds found in the Khat plant, native to eastern Africa.
Substituted cathinones, or simply cathinones, which include some stimulants and entactogens, are derivatives of cathinone. They feature a phenethylamine core with an alkyl group attached to the alpha carbon, and a ketone group attached to the beta carbon, along with additional substitutions. Cathinone occurs naturally in the plant khat whose leaves are chewed as a recreational drug.

3-Methoxyphencyclidine (3-MeO-PCP) is a dissociative hallucinogen of the arylcyclohexylamine class related to phencyclidine (PCP) which has been sold online as a designer drug. It has been used across Europe and the United States. In some cases, consumption has been known to be fatal. It acts mainly as an NMDA receptor antagonist, though it has also been found to interact with the sigma σ1 receptor and the serotonin transporter. The drug does not possess any opioid activity nor does it act as a dopamine reuptake inhibitor.

4-Methoxyphencyclidine is a dissociative anesthetic drug that has been sold online as a research chemical. The synthesis of 4-MeO-PCP was first reported in 1965 by the Parke-Davis medicinal chemist Victor Maddox. A 1999 review published by a chemist using the pseudonym John Q. Beagle suggested the potency of 4-MeO-PCP in man was reduced relative to PCP, two years later Beagle published a detailed description of the synthesis and qualitative effects of 4-MeO-PCP, which he said possessed 70% the potency of PCP. 4-MeO-PCP was the first arylcyclohexylamine research chemical to be sold online, it was introduced in late 2008 by a company trading under the name CBAY and was followed by several related compounds such as 3-MeO-PCP and methoxetamine. 4-MeO-PCP has lower affinity for the NMDA receptor than PCP, but higher affinity than ketamine, it is orally active in a dosage range similar to ketamine, with some users requiring doses in excess of 100 mg for desired effects. Users have reported substantial differences in active dose, these discrepancies can be partially explained by the presence of unreacted PCC and other impurities in samples sold on the grey market. 4-MeO-PCP has Ki values of 404 nM for the NMDA receptor, 713 nM for the norepinephrine transporter, 844 nM for the serotonin transporter, 296 nM for the σ1 receptor and 143 nM for the σ2 receptor.
6-APB is an empathogenic psychoactive drug of the substituted benzofuran and substituted phenethylamine classes. 6-APB and other compounds are sometimes informally called "Benzofury" in newspaper reports. It is similar in structure to MDA, but differs in that the 3,4-methylenedioxyphenyl ring system has been replaced with a benzofuran ring. 6-APB is also the unsaturated benzofuran derivative of 6-APDB. It may appear as a tan grainy powder.

Methoxetamine (MXE) is a dissociative hallucinogen that has been sold as a designer drug. It differs from many dissociatives such as ketamine and phencyclidine (PCP) that were developed as pharmaceutical drugs for use as general anesthetics in that it was designed specifically to increase the antidepressant effects of ketamine.
A temporary class drug is a relatively new status for controlled drugs, which has been adopted in some jurisdictions, notably New Zealand and the United Kingdom, to attempt to bring newly synthesised designer drugs under legal control. The controlled drug legislation in these jurisdictions requires drug scheduling decisions to follow an evidence-based process, where the harms of the drug are assessed and reviewed so that an appropriate legal status can be assigned. Since many designer drugs sold in recent years have had little or no published research that could help inform such a decision, they have been widely sold as "legal highs", often for months, before sufficient evidence accumulates to justify placing them on the controlled drug schedules.

Bath salts are a group of recreational designer drugs. The name derives from instances in which the drugs were disguised as bath salts. The white powder, granules, or crystals often resemble Epsom salts, but differ chemically. The drugs' packaging often states "not for human consumption" in an attempt to circumvent drug prohibition laws. Additionally, they may be described as "plant food", "powdered cleaner", or other products.

Diphenyl-2-pyridylmethane is a triaryl organic compound that has been used to selectively extract specific metal ions into organic solvents. Its pharmacology is similar to the stimulant desoxypipradrol in which the pyridine ring is reduced to a piperidine and for which it is a chemical precursor.

4,4'-Dimethylaminorex, sometimes referred to by the street name "Serotoni", is a psychostimulant and entactogen designer drug related to aminorex, 4-methylaminorex, and pemoline. It was first detected in the Netherlands in December 2012, and has been sold as a designer drug around Europe since mid-2013.