
Monoamine transporters (MATs) are protein structures that function as integral plasma-membrane transporters to regulate concentrations of extracellular monoamine neurotransmitters. Three major classes of MATs are responsible for the reuptake of their associated amine neurotransmitters. MATs are located just outside the synaptic cleft (peri-synaptically), transporting monoamine transmitter overflow from the synaptic cleft back to the cytoplasm of the pre-synaptic neuron. MAT regulation generally occurs through protein phosphorylation and posttranslational modification. Due to their significance in neuronal signaling, MATs are commonly associated with drugs used to treat mental disorders as well as recreational drugs. Compounds targeting MATs range from medications such as the wide variety of tricyclic antidepressants, selective serotonin reuptake inhibitors such as fluoxetine (Prozac) to stimulant medications such as methylphenidate (Ritalin) and amphetamine in its many forms and derivatives methamphetamine (Desoxyn) and lisdexamfetamine (Vyvanse). Furthermore, drugs such as MDMA and natural alkaloids such as cocaine exert their effects in part by their interaction with MATs, by blocking the transporters from mopping up dopamine, serotonin, and other neurotransmitters from the synapse.

Serotonin–norepinephrine reuptake inhibitors (SNRIs) are a class of antidepressant drugs used to treat major depressive disorder (MDD), anxiety disorders, obsessive–compulsive disorder (OCD), social phobia, attention-deficit hyperactivity disorder (ADHD), chronic neuropathic pain, fibromyalgia syndrome (FMS), and menopausal symptoms. SNRIs are monoamine reuptake inhibitors; specifically, they inhibit the reuptake of serotonin and norepinephrine. These neurotransmitters are thought to play an important role in mood regulation. SNRIs can be contrasted with the more widely used selective serotonin reuptake inhibitors (SSRIs), which act upon serotonin only.

A norepinephrine reuptake inhibitor or noradrenaline reuptake inhibitor or adrenergic reuptake inhibitor (ARI), is a type of drug that acts as a reuptake inhibitor for the neurotransmitters norepinephrine (noradrenaline) and epinephrine (adrenaline) by blocking the action of the norepinephrine transporter (NET). This in turn leads to increased extracellular concentrations of norepinephrine and epinephrine and therefore can increase adrenergic neurotransmission.
Phenyltropanes (PTs) were originally developed to reduce cocaine addiction and dependency. In general these compounds act as inhibitors of the plasmalemmal monoamine reuptake transporters. Although RTI holds a strong position in this field, they are not the only researchers that have prepared these analogues. This research has spanned beyond the last couple decades, and has picked up its pace in recent times, creating numerous phenyltropanes as research into cocaine analogues garners interest to treat addiction.

(+)-CPCA is a stimulant drug similar in structure to pethidine and to RTI-31, but nocaine is lacking the two-carbon bridge of RTI-31's tropane skeleton. This compound was first developed as a substitute agent for cocaine.
A serotonin–norepinephrine–dopamine reuptake inhibitor (SNDRI), also known as a triple reuptake inhibitor (TRI), is a type of drug that acts as a combined reuptake inhibitor of the monoamine neurotransmitters serotonin, norepinephrine, and dopamine. It does this by concomitantly inhibiting the serotonin transporter (SERT), norepinephrine transporter (NET), and dopamine transporter (DAT), respectively. Inhibition of the reuptake of these neurotransmitters increases their extracellular concentrations and, therefore, results in an increase in serotonergic, adrenergic, and dopaminergic neurotransmission.

A serotonin reuptake inhibitor (SRI) is a type of drug which acts as a reuptake inhibitor of the neurotransmitter serotonin by blocking the action of the serotonin transporter (SERT). This in turn leads to increased extracellular concentrations of serotonin and, therefore, an increase in serotonergic neurotransmission. It is a type of monoamine reuptake inhibitor (MRI); other types of MRIs include dopamine reuptake inhibitors and norepinephrine reuptake inhibitors.

Tesofensine (NS2330) is a serotonin–noradrenaline–dopamine reuptake inhibitor from the phenyltropane family of drugs, which is being developed for the treatment of obesity. Tesofensine was originally developed by a Danish biotechnology company, NeuroSearch, who transferred the rights to Saniona in 2014.

A reuptake inhibitor (RI) is a type of drug known as a reuptake modulator that inhibits the plasmalemmal transporter-mediated reuptake of a neurotransmitter from the synapse into the pre-synaptic neuron. This leads to an increase in extracellular concentrations of the neurotransmitter and an increase in neurotransmission. Various drugs exert their psychological and physiological effects through reuptake inhibition, including many antidepressants and psychostimulants.

Diclofensine was developed by Hoffmann-La Roche in the 1970s in the search for a new antidepressant. It was found that the (S)-isomer was responsible for activity. Is a stimulant drug which acts as a triple monoamine reuptake inhibitor, primarily inhibiting the reuptake of dopamine and norepinephrine, with affinities (Ki) of 16.8 nM, 15.7 nM, and 51 nM for DAT, NET, and SERT, respectively. It was found to be an effective antidepressant in human trials, with relatively few side effects, but was ultimately dropped from clinical development, possibly due to concerns about its abuse potential.

Dichloropane ((−)-2β-Carbomethoxy-3β-(3,4-dichlorophenyl)tropane, RTI-111, O-401) is a stimulant of the phenyltropane class that acts as a serotonin–norepinephrine–dopamine reuptake inhibitor (SNDRI) with IC50 values of 3.13, 0.79 and 18 nM, respectively. In animal studies, dichloropane had a slower onset and longer duration of action compared to cocaine.

NS-2359 (GSK-372,475) is a serotonin-norepinephrine-dopamine reuptake inhibitor. It was under development by GlaxoSmithKline (GSK) as an antidepressant, but was discontinued in 2009 when phase II clinical trials showed the drug was not effective and not well tolerated. The results did not support further effort by the company. NS-2359 was also in clinical trials for the treatment of ADHD, phase II having been completed in 2007. A phase I clinical trial exploring the effect of NS-2359 on cocaine-dependent individuals was completed in 2002.
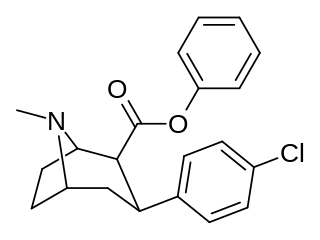
RTI(-4229)-113 is a stimulant drug which acts as a potent and fully selective dopamine reuptake inhibitor (DRI). It has been suggested as a possible substitute drug for the treatment of cocaine addiction. "RTI-113 has properties that make it an ideal medication for cocaine abusers, such as an equivalent efficacy, a higher potency, and a longer duration of action as compared to cocaine." Replacing the methyl ester in RTI-31 with a phenyl ester makes the resultant RTI-113 fully DAT specific. RTI-113 is a particularly relevant phenyltropane cocaine analog that has been tested on squirrel monkeys. RTI-113 has also been tested against cocaine in self-administration studies for DAT occupancy by PET on awake rhesus monkeys. The efficacy of cocaine analogs to elicit self-administration is closely related to the rate at which they are administered. Slower onset of action analogs are less likely to function as positive reinforcers than analogues that have a faster rate of onset.

RTI(-4229)-112 is a synthetic stimulant drug from the phenyltropane family. In contrast to RTI-113, which is DAT selective, RTI-112 is a nonselective triple reuptake inhibitor.

JNJ-7925476 is a triple reuptake inhibitor antidepressant discovered by Johnson & Johnson, but never marketed.

JZ-IV-10 is a piperidine derivative related to cocaine which acts as a highly potent serotonin–norepinephrine–dopamine reuptake inhibitor. The eugeroic modafinil was used as a lead to fuel this compound's discovery.

A serotonin–dopamine reuptake inhibitor (SDRI) is a type of drug which acts as a reuptake inhibitor of the monoamine neurotransmitters serotonin and dopamine by blocking the actions of the serotonin transporter (SERT) and dopamine transporter (DAT), respectively. This in turn leads to increased extracellular concentrations of serotonin and dopamine, and, therefore, an increase in serotonergic and dopaminergic neurotransmission.
A monoamine reuptake inhibitor (MRI) is a drug that acts as a reuptake inhibitor of one or more of the three major monoamine neurotransmitters serotonin, norepinephrine, and dopamine by blocking the action of one or more of the respective monoamine transporters (MATs), which include the serotonin transporter (SERT), norepinephrine transporter (NET), and dopamine transporter (DAT). This in turn results in an increase in the synaptic concentrations of one or more of these neurotransmitters and therefore an increase in monoaminergic neurotransmission.

Decynium-22 is a cationic derivative of quinoline, and a potent inhibitor of the plasma membrane monoamine transporter (PMAT), as well as all members of the organic cation transporter (OCT) family in both human and rat cells. However, it has little effect on high affinity monoamine transporters such as the dopamine transporter and norepinephrine transporter.
Selective norepinephrine reuptake inhibitors (sNRIs) are a class of drugs that have been marketed as antidepressants and are used for various mental disorders, mainly depression and attention-deficit hyperactivity disorder (ADHD). The norepinephrine transporter (NET) serves as the fundamental mechanism for the inactivation of noradrenergic signaling because of the NET termination in the reuptake of norepinephrine (NE). The selectivity and mechanism of action for the NRI drugs remain mostly unresolved and, to date, only a limited number of NRI-selective inhibitors are available. The first commercially available selective NRI was the drug reboxetine (Edronax), developed as a first-line therapy for major depressive disorder. Atomoxetine (Strattera) is another potent and selective NRI which is also effective and well tolerated for the treatment of ADHD in adults; it may also be a new treatment option for adults with ADHD, particularly for those patients at risk of substance abuse.


















