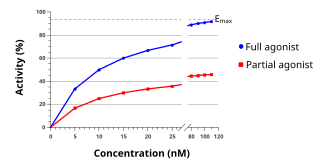
An agonist is a chemical that activates a receptor to produce a biological response. Receptors are cellular proteins whose activation causes the cell to modify what it is currently doing. In contrast, an antagonist blocks the action of the agonist, while an inverse agonist causes an action opposite to that of the agonist.

A receptor antagonist is a type of receptor ligand or drug that blocks or dampens a biological response by binding to and blocking a receptor rather than activating it like an agonist. Antagonist drugs interfere in the natural operation of receptor proteins. They are sometimes called blockers; examples include alpha blockers, beta blockers, and calcium channel blockers. In pharmacology, antagonists have affinity but no efficacy for their cognate receptors, and binding will disrupt the interaction and inhibit the function of an agonist or inverse agonist at receptors. Antagonists mediate their effects by binding to the active site or to the allosteric site on a receptor, or they may interact at unique binding sites not normally involved in the biological regulation of the receptor's activity. Antagonist activity may be reversible or irreversible depending on the longevity of the antagonist–receptor complex, which, in turn, depends on the nature of antagonist–receptor binding. The majority of drug antagonists achieve their potency by competing with endogenous ligands or substrates at structurally defined binding sites on receptors.

5-HT receptors, 5-hydroxytryptamine receptors, or serotonin receptors, are a group of G protein-coupled receptor and ligand-gated ion channels found in the central and peripheral nervous systems. They mediate both excitatory and inhibitory neurotransmission. The serotonin receptors are activated by the neurotransmitter serotonin, which acts as their natural ligand.
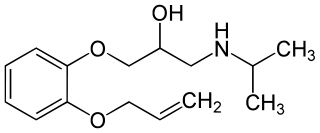
Oxprenolol is a non-selective beta blocker with some intrinsic sympathomimetic activity. It is used for the treatment of angina pectoris, abnormal heart rhythms and high blood pressure.

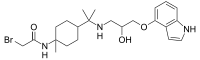
Pindolol, sold under the brand name Visken among others, is a nonselective beta blocker which is used in the treatment of hypertension. It is also an antagonist of the serotonin 5-HT1A receptor, preferentially blocking inhibitory 5-HT1A autoreceptors, and has been researched as an add-on therapy to various antidepressants, such as clomipramine and the selective serotonin reuptake inhibitors (SSRIs), in the treatment of depression and obsessive-compulsive disorder.

Etoperidone, associated with several brand names, is an atypical antidepressant which was developed in the 1970s and either is no longer marketed or was never marketed. It is a phenylpiperazine related to trazodone and nefazodone in chemical structure and is a serotonin antagonist and reuptake inhibitor (SARI) similarly to them.

The serotonin 1A receptor is a subtype of serotonin receptors, or 5-HT receptors, that binds serotonin, also known as 5-HT, a neurotransmitter. 5-HT1A is expressed in the brain, spleen, and neonatal kidney. It is a G protein-coupled receptor (GPCR), coupled to the Gi protein, and its activation in the brain mediates hyperpolarization and reduction of firing rate of the postsynaptic neuron. In humans, the serotonin 1A receptor is encoded by the HTR1A gene.

Tertatolol is a medication in the class of beta blockers, used in the treatment of high blood pressure. It was discovered by the French pharmaceutical company Servier and is marketed in Europe.

WAY-100635 is a piperazine drug and research chemical widely used in scientific studies. It was originally believed to act as a selective 5-HT1A receptor antagonist, but subsequent research showed that it also acts as potent full agonist at the D4 receptor. It is sometimes referred to as a silent antagonist at the former receptor. It is closely related to WAY-100135.

Flesinoxan (DU-29,373) is a potent and selective 5-HT1A receptor partial/near-full agonist of the phenylpiperazine class. Originally developed as a potential antihypertensive drug, flesinoxan was later found to possess antidepressant and anxiolytic effects in animal tests. As a result, it was investigated in several small human pilot studies for the treatment of major depressive disorder, and was found to have robust effectiveness and very good tolerability. However, due to "management decisions", the development of flesinoxan was stopped and it was not pursued any further.

Isamoltane (CGP-361A) is a drug used in scientific research. It acts as an antagonist at the β-adrenergic, 5-HT1A, and 5-HT1B receptors. It has about five times the potency for the 5-HT1B receptor over the 5-HT1A receptor. It has anxiolytic effects in rodents.
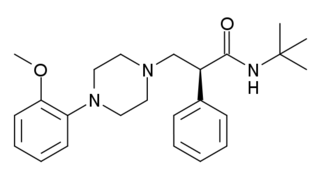
WAY-100135 is a serotonergic drug of the phenylpiperazine family which is used in scientific research. It acts as potent 5-HT1A receptor antagonist, and was originally believed to be highly selective, but further studies have demonstrated that it also acts as a partial agonist of the 5-HT1D receptor (pKi = 7.58; virtually the same affinity for 5-HT1A), and to a much lesser extent, of the 5-HT1B receptor (pKi = 5.82). These findings may have prompted the development of the related compound WAY-100635, another purportedly selective and even more potent 5-HT1A antagonist, which was synthesized shortly thereafter. However, WAY-100635 turned out to be non-selective as well, having been shown to act additionally as a potent D4 receptor agonist later on.
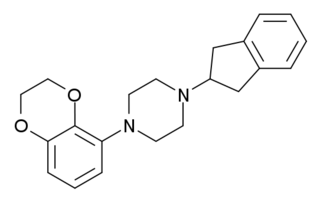
S-15535 is a phenylpiperazine drug which is a potent and highly selective 5-HT1A receptor ligand that acts as an agonist and antagonist at the presynaptic and postsynaptic 5-HT1A receptors, respectively. It has anxiolytic properties.
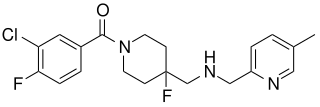
Befiradol is an experimental drug being studied for the treatment of levodopa-induced dyskinesia. It is a potent and selective 5-HT1A receptor full agonist.

Serotonin antagonist and reuptake inhibitors (SARIs) are a class of drugs used mainly as antidepressants, but also as anxiolytics and hypnotics. They act by antagonizing serotonin receptors such as 5-HT2A and inhibiting the reuptake of serotonin, norepinephrine, and/or dopamine. Additionally, most also antagonize α1-adrenergic receptors. The majority of the currently marketed SARIs belong to the phenylpiperazine class of compounds.

Adatanserin is a mixed 5-HT1A receptor partial agonist and 5-HT2A and 5-HT2C receptor antagonist. It was under development by Wyeth as an antidepressant but was ultimately not pursued.

F-15,599, also known as NLX-101, is a potent and selective 5-HT1A receptor full agonist. It displays functional selectivity by strongly activating 5-HT1A receptors in the postsynaptic prefrontal cortex while having little effect on somatodendritic autoreceptors in the raphe nucleus. As a result, it has been touted as a preferential postsynaptic 5-HT1A receptor agonist and has been investigated as a novel potential antidepressant.

Repinotan (BAYx3702), an aminomethylchroman derivative, is a selective 5-HT1A receptor full agonist with high potency and efficacy. It has neuroprotective effects in animal studies, and was trialed in humans for reducing brain injury following head trauma. It was subsequently trialed up to phase II for treatment of stroke, but while side effects were mild and consisted mainly of nausea, repinotan failed to demonstrate sufficient efficacy to justify further clinical trials. However, repinotan continues to be investigated for other applications, and was found to be effective at counteracting the respiratory depression produced by morphine, though with slight reduction in analgesic effects.

Mefway is a serotonin 5-HT1A receptor antagonist used in medical research, usually in the form of mefway (18F) as a positron emission tomography (PET) radiotracer.

F-15,063 is an orally active potential antipsychotic, and an antagonist at the D2/D3 receptors, partial agonist at the D4 receptor, and agonist at the 5-HT1A receptors. It has greater efficacy at the 5-HT1A receptors than other antipsychotics, such as clozapine, aripiprazole, and ziprasidone. This greater efficacy may lead to enhanced antipsychotic properties, as antipsychotics that lack 5-HT1A affinity are associated with increased risk of extrapyramidal symptoms, and lack of activity against the negative symptoms of schizophrenia.