
Memantine is a medication used to slow the progression of moderate-to-severe Alzheimer's disease. It is taken by mouth.
Neurocrine Biosciences, Inc. is an American biopharmaceutical company founded in 1992. The company is headquartered in San Diego, California, and led by CEO Kevin Gorman. Neurocrine develops treatments for neurological and endocrine-related diseases and disorders. In 2017, the company's drug valbenazine (Ingrezza) was approved in the US to treat adults with tardive dyskinesia (TD).

Latrepirdine is an antihistamine drug which has been used clinically in Russia since 1983.

The 5HT6 receptor is a subtype of 5HT receptor that binds the endogenous neurotransmitter serotonin (5-hydroxytryptamine, 5HT). It is a G protein-coupled receptor (GPCR) that is coupled to Gs and mediates excitatory neurotransmission. HTR6 denotes the human gene encoding for the receptor.
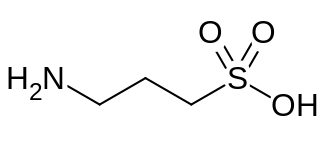
Homotaurine is a natural sulfonic acid found in seaweed. It is analogous to taurine, but with an extra carbon in its chain. It has GABAergic activity, apparently by mimicking GABA, which it resembles.

Casopitant (INN), former tentative trade names Rezonic (U.S.) and Zunrisa (Europe), is an NK1 receptor antagonist which was undergoing research for the treatment of chemotherapy-induced nausea and vomiting. It was under development by GlaxoSmithKline. In July 2008, the company filed a marketing authorisation application with the European Medicines Agency. The application was withdrawn and development was discontinued in September 2009 because GlaxoSmithKline decided that further safety assessment was necessary. However, a 2022 review listed casopitant as under development as a potential novel antidepressant for the treatment of major depressive disorder, with a phase 2 clinical trial having been completed.

Lecozotan is an investigational drug by Wyeth tested for improvement of cognitive functions of Alzheimer's disease patients. As of June 2008, the first Phase III clinical trial has been completed.

Pimavanserin, sold under the brand name Nuplazid, is an atypical antipsychotic which is approved for the treatment of Parkinson's disease psychosis and is also being studied for the treatment of Alzheimer’s disease psychosis, schizophrenia, agitation, and major depressive disorder. Unlike other antipsychotics, pimavanserin is not a dopamine receptor antagonist.

Ecopipam is a dopamine antagonist which is under development for the treatment of Lesch-Nyhan syndrome, Tourette's syndrome, speech disorders, and restless legs syndrome. It is taken by mouth.
Idalopirdine (INN) (code names Lu AE58054,) is a potent and selective 5-HT6 receptor antagonist under development by Lundbeck as an augmentation therapy for the treatment of cognitive deficits associated with Alzheimer's disease and schizophrenia. As of October 2013 it is in phase III clinical trials.
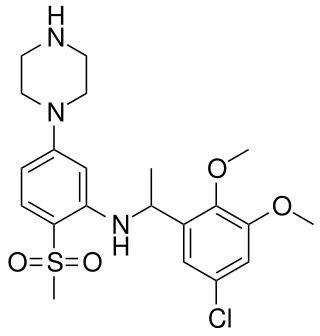
PRX-07034 is a selective 5-HT6 receptor antagonist. It has cognition and memory-enhancing properties and potently decreases food intake and body weight in rodents. PRX-07034 was under development by Epix Pharmaceuticals for the treatment of obesity and cognitive impairment associated with Alzheimer's disease and schizophrenia but upon the company collapsing due to lack of funds the compound was auctioned to another corporation.

Cerlapirdine is a drug which was under development by Wyeth/Pfizer for the treatment of cognitive disorders associated with Alzheimer's disease and schizophrenia. In a phase II clinical trial it demonstrated a trend toward efficacy along with a good side effect profile and no incidence of serious adverse events, but no further development has occurred since 2011.

Brexpiprazole, sold under the brand name Rexulti among others, is a medication used for the treatment of major depressive disorder, schizophrenia, and agitation associated with dementia due to Alzheimer's disease. It is an atypical antipsychotic.
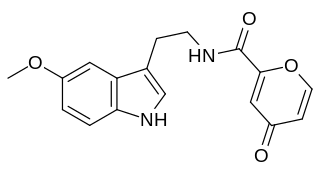
Piromelatine (Neu-P11) is a multimodal sleep drug under development by Neurim Pharmaceuticals. It is an agonist at melatonin MT1/MT2 and serotonin 5-HT1A/5-HT1D receptors. Neurim is conducting a phase II randomized, placebo controlled trial of cognitive and sleep effects in Alzheimer's disease.

AVN-211 (CD-008-0173) is a drug which acts as a highly selective 5-HT6 receptor antagonist and is under development by Avineuro Pharmaceuticals for the treatment of schizophrenia. In early 2011, it successfully completed phase IIa clinical trials, with benefits on positive symptoms and some procognitive effects observed, and in mid 2013, phase IIb clinical trials for schizophrenia began. Avineuro Pharmaceuticals also expressed intention to start clinical trials of AVN-211 for Alzheimer's disease in 2015.

Brilaroxazine, also known as oxaripiprazole, is an investigational atypical antipsychotic which is under development by Reviva Pharmaceuticals for the treatment neuropsychiatric and inflammatory disorders. As of July 2023, it is in phase III clinical trials for schizophrenia. Reviva Pharmaceuticals also intends to investigate brilaroxazine for the treatment of bipolar disorder, major depressive disorder, attention deficit hyperactivity disorder (ADD/ADHD), psychosis/agitation associated with Alzheimer's disease, Parkinson's disease psychosis, as well as the inflammatory disorders pulmonary arterial hypertension (PAH), idiopathic pulmonary fibrosis (IPF), and psoriasis. The FDA granted brilaroxazine orphan drug designation for the treatment of PAH and IPF.

AVN-101, a close structural analogue of latrepirdine, is a selective 5-HT6 receptor antagonist which is under development by Avineuro Pharmaceuticals for the treatment of Alzheimer's disease and anxiety disorders. As of November 2013, it was in phase II clinical trials for these indications.

Sio Gene Therapies, formerly known as Axovant Gene Therapies, is a clinical-stage pharmaceutical company that develops gene therapies to treat neurological disorders. The company is headquartered in New York City and is incorporated in Basel, Switzerland. The company was founded by former hedge fund analyst Vivek Ramaswamy in 2014 as a wholly owned subsidiary of Roivant Sciences.

SAGE-718 is experimental drug being investigated for the treatment of neurological disorders and cognitive impairment. It acts as a positive allosteric modulator of the NMDA receptor, whose activity is essential for learning, memory, and cognition. SAGE-718 is an analog of the neurosteroid 24S-hydroxycholesterol.

AVN-322 is a 5-hydroxytryptamine subtype 6 receptor antagonist manufactured by Avineuro Pharmaceuticals Inc. that could potentially be used to combat Alzheimer's disease and schizophrenia. AVN-322 also reverses the negative effects of scopolamine and MK-80.


















