Related Research Articles
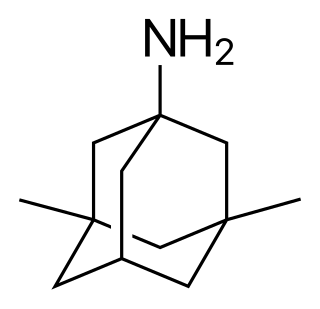
Memantine, sold under the brand name Namenda among others, is a medication used to slow the progression of moderate-to-severe Alzheimer's disease. It is taken by mouth.

CX717 is an ampakine compound created by Christopher Marrs and Gary Rogers in 1996 at Cortex Pharmaceuticals. It affects the neurotransmitter glutamate, with trials showing the drug improves cognitive functioning and memory.

Monoclonal antibodies (mAbs) have varied therapeutic uses. It is possible to create a mAb that binds specifically to almost any extracellular target, such as cell surface proteins and cytokines. They can be used to render their target ineffective, to induce a specific cell signal, to cause the immune system to attack specific cells, or to bring a drug to a specific cell type.
Bapineuzumab is a humanized monoclonal antibody that acts on the nervous system and may have potential therapeutic value for the treatment of Alzheimer's disease and possibly glaucoma. However, in 2012 it failed to produce significant cognitive improvements in patients in two major trials, despite lowering key biomarkers of AD, amyloid brain plaque and hyperphosphorylated tau protein in CSF.

Latrepirdine is an antihistamine drug which has been used clinically in Russia since 1983.
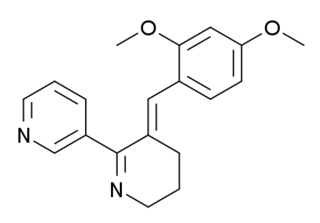
GTS-21 is a drug that has been shown to enhance memory and cognitive function. It has been studied for its potential therapeutic uses, particularly in the treatment of neurodegenerative diseases and psychiatric disorders.

Xanomeline is a small molecule muscarinic acetylcholine receptor agonist that was first synthesized in a collaboration between Eli Lilly and Novo Nordisk as an investigational therapeutic being studied for the treatment of central nervous system (CNS) disorders.

Pimavanserin, sold under the brand name Nuplazid, is an atypical antipsychotic which is approved for the treatment of Parkinson's disease psychosis. Unlike other antipsychotics, pimavanserin is not a dopamine receptor antagonist, but rather is a selective inverse agonist of the serotonin 5-HT2A receptor.

Intepirdine (INN; developmental codes SB-742457, RVT-101) is a selective 5-HT6 receptor antagonist with potential cognition, memory, and learning-enhancing effects. It was under development by GlaxoSmithKline for the treatment of Alzheimer's disease and demonstrated some preliminary efficacy in phase II clinical trials. GSK chose not to continue development and sold the rights to Axovant Sciences for $5 million in December 2014.
Trevena, Inc. is a clinical stage biopharmaceutical company, headquartered in Chesterbrook, Pennsylvania, USA, and is involved in the discovery and development of G-protein coupled receptors (GPCR) biased ligands. Trevena was founded in 2007 with technology licensed from Duke University, which originated in the labs of company founders Robert Lefkowitz winner of the 2012 Nobel Prize in Chemistry and Howard Rockman. Trevena's approach to drug discovery is based on utilizing ligand bias, or functional selectivity, at GPCR targets to produce drugs with improved efficacy and reduced side effect profiles. Trevena was named one of the top 15 US startups of 2008 by Business Week.
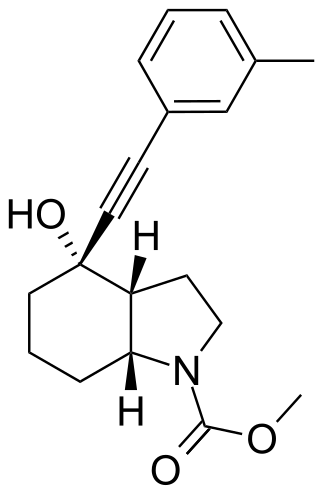
Mavoglurant (developmental code name AFQ-056) is an experimental drug candidate for the treatment of fragile X syndrome and other conditions. It exerts its effect as an antagonist of the metabotropic glutamate receptor 5 (mGluR5).

Brexpiprazole, sold under the brand name Rexulti among others, is an atypical antipsychotic medication used for the treatment of major depressive disorder, schizophrenia, and agitation associated with dementia due to Alzheimer's disease.
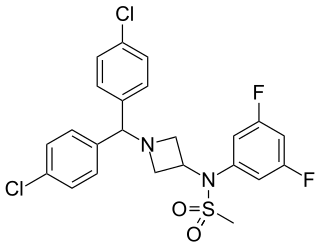
Drinabant (INN; AVE-1625) is a drug that acts as a selective CB1 receptor antagonist, which was under investigation varyingly by Sanofi-Aventis as a treatment for obesity, schizophrenia, Alzheimer's disease, Parkinson's disease, and nicotine dependence. Though initially studied as a potential treatment for a variety of different medical conditions, Sanofi-Aventis eventually narrowed down the therapeutic indications of the compound to just appetite suppression. Drinabant reached phase IIb clinical trials for this purpose in the treatment of obesity but was shortly thereafter discontinued, likely due to the observation of severe psychiatric side effects including anxiety, depression, and thoughts of suicide in patients treated with the now-withdrawn rimonabant, another CB1 antagonist that was also under development by Sanofi-Aventis.
Piromelatine (Neu-P11) is a multimodal sleep drug under development by Neurim Pharmaceuticals. It is an agonist at melatonin MT1/MT2 and serotonin 5-HT1A/5-HT1D receptors. Neurim is conducting a phase II randomized, placebo controlled trial of cognitive and sleep effects in Alzheimer's disease.

AVN-211 (CD-008-0173) is a drug which acts as a highly selective 5-HT6 receptor antagonist and is under development by Avineuro Pharmaceuticals for the treatment of schizophrenia. In early 2011, it successfully completed phase IIa clinical trials, with benefits on positive symptoms and some procognitive effects observed, and in mid 2013, phase IIb clinical trials for schizophrenia began. Avineuro Pharmaceuticals also expressed intention to start clinical trials of AVN-211 for Alzheimer's disease in 2015.

Maritupirdine (developmental code name AVN-101), a close structural analogue of latrepirdine, is a selective 5-HT6 receptor antagonist which is under development by Avineuro Pharmaceuticals for the treatment of Alzheimer's disease and anxiety disorders. As of November 2013, it was in phase II clinical trials for these indications.

Blarcamesine is an experimental drug which is under development for the treatment of Alzheimer's disease and a variety of other indications.

Dextromethorphan/bupropion (DXM/BUP), sold under the brand name Auvelity, is a combination medication for the treatment of major depressive disorder (MDD). Its active components are dextromethorphan (DXM) and bupropion. Patients who stayed on the medication had an average of 11% greater reduction in depressive symptoms than placebo in an FDA approval trial. It is taken as a tablet by mouth.

Simufilam (PTI-125) is an experimental medication for the treatment of Alzheimer's disease. It is being developed by the American pharmaceutical firm Cassava Sciences. The drug is in phase III clinical trials as of October 2023. There are two phase III clinical studies: RETHINK-ALZ, a 52-week trial, is set to complete in 2024, and REFOCUS-ALZ, spanning 76 weeks, is projected to finish in 2025.

AVN-322 is a 5-hydroxytryptamine subtype 6 receptor antagonist manufactured by Avineuro Pharmaceuticals Inc. that could potentially be used to combat Alzheimer's disease and schizophrenia. AVN-322 also reverses the negative effects of scopolamine and MK-80.
References
- ↑ Griebel, Guy; Holmes, Andrew (2013). "50 years of hurdles and hope in anxiolytic drug discovery". Nature Reviews Drug Discovery. 12 (9): 667–687. doi:10.1038/nrd4075. ISSN 1474-1776. PMC 4176700 . PMID 23989795. S2CID 6531810.
- ↑ "AVN 397 - AdisInsight". adisinsight.springer.com. Retrieved 2023-03-12.
- ↑ "Avineuro Announces Beginning Of Phase II Clinical Studies Of AVN-397, Potent Small Molecule For Treatment Of Anxiety And Alzheimer's Disease". BioSpace. Retrieved 2023-03-12.
- ↑ Froestl, W.; Pfeifer, A.; Muhs, A. (2014). "Cognitive enhancers (Nootropics). Part 3: drugs interacting with targets other than receptors or enzymes. Disease-modifying drugs. Update 2014". Journal of Alzheimer's Disease. 42 (4): 1079–1149. doi:10.3233/JAD-141206. PMID 25061058. S2CID 998047 . Retrieved 2023-03-12.
- ↑ Medicines in Development / Mental Illnesses / 2012 Report (PDF), archived (PDF) from the original on 9 Jul 2022