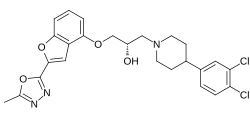
Vilazodone, sold under the brand name Viibryd among others, is a medication used to treat major depressive disorder. It is classified as a serotonin modulator and is taken by mouth.
Scientific studies have found that different brain areas show altered activity in humans with major depressive disorder (MDD), and this has encouraged advocates of various theories that seek to identify a biochemical origin of the disease, as opposed to theories that emphasize psychological or situational causes. Factors spanning these causative groups include nutritional deficiencies in magnesium, vitamin D, and tryptophan with situational origin but biological impact. Several theories concerning the biologically based cause of depression have been suggested over the years, including theories revolving around monoamine neurotransmitters, neuroplasticity, neurogenesis, inflammation and the circadian rhythm. Physical illnesses, including hypothyroidism and mitochondrial disease, can also trigger depressive symptoms.

Vortioxetine, sold under the brand names Trintellix and Brintellix among others, is a medication used to treat major depressive disorder. Its effectiveness is viewed as similar to that of other antidepressants. It is taken by mouth.

Tedatioxetine is an experimental antidepressant that was discovered by scientists at Lundbeck; in 2007 Lundbeck and Takeda entered into a partnership that included tedatioxetine but was focused on another, more advanced Lundbeck drug candidate, vortioxetine.

Brexpiprazole, sold under the brand name Rexulti among others, is a medication used for the treatment of major depressive disorder, schizophrenia, and agitation associated with dementia due to Alzheimer's disease. It is an atypical antipsychotic.

Rapastinel is a novel antidepressant that was under development by Allergan as an adjunctive therapy for the treatment of treatment-resistant depression. It is a centrally active, intravenously administered amidated tetrapeptide that acts as a novel and selective modulator of the NMDA receptor. The drug is a rapid-acting and long-lasting antidepressant as well as robust cognitive enhancer by virtue of its ability to enhance NMDA receptor-mediated signal transduction and synaptic plasticity.

NSI-189 is an experimental, potential antidepressant that was developed by Neuralstem, Inc. for the treatment for major depressive disorder (MDD), as well as for cognitive impairment and neurodegeneration.

Apimostinel is an investigational antidepressant, acting as a novel and selective modulator of the NMDA receptor. It is currently under development for the acute treatment of major depressive disorder (MDD) by Gate Neurosciences, and previously by Naurex and Allergan. As of February 2015, an intravenous formulation of apimostinel has completed a phase IIa clinical trial for MDD.
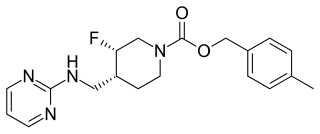
Rislenemdaz is an orally active, selective NMDA receptor subunit 2B (NR2B) antagonist which is under development by Cerecor in the United States as an adjunctive therapy for treatment-resistant depression (TRD). In November 2013, phase II clinical trials were initiated, and in the same month, rislenemdaz received Fast Track Designation from the Food and Drug Administration for TRD.
TGBA01AD (also known as FKB01MD) is a serotonin reuptake inhibitor, 5-HT1A and 5-HT1D receptor agonist, and 5-HT2 receptor antagonist which is under development by Fabre-Kramer for the treatment of major depressive disorder. It has been in phase II clinical trials since 2009, and as of January 2016, remains in this phase of development.

Seltorexant, also known by its developmental code names MIN-202 and JNJ-42847922, is an orexin antagonist medication which is under development for the treatment of depression and insomnia. It is a selective antagonist of the orexin OX2 receptor (2-SORA). The medication is taken by mouth. As of February 2022, seltorexant is in phase 3 clinical trials for treatment of major depressive disorder (MDD) and phase 2 trials for treatment of insomnia. It was also under investigation for the treatment of sleep apnea, but no recent development has been reported for this indication. Seltorexant is under development by Minerva Neurosciences and Johnson & Johnson's Janssen Pharmaceuticals.

BTRX-246040, also known as LY-2940094, is a potent and selective nociceptin receptor antagonist which is under development by BlackThorn Therapeutics and Eli Lilly for the treatment of major depressive disorder (MDD). It has demonstrated proof-of-concept clinical efficacy for depression. As of 2017, it is in phase II clinical trials for the treatment of MDD. It was also under investigation for the treatment of alcoholism, and similarly reached phase II clinical studies for this indication, but development was discontinued.

Brilaroxazine, also known as oxaripiprazole, is an investigational atypical antipsychotic which is under development by Reviva Pharmaceuticals for the treatment of neuropsychiatric and inflammatory disorders. It has currently completed the first of two phase III clinical trials for schizophrenia. Reviva Pharmaceuticals also intends to investigate brilaroxazine for the treatment of bipolar disorder, major depressive disorder, attention deficit hyperactivity disorder (ADD/ADHD), psychosis/agitation associated with Alzheimer's disease, Parkinson's disease psychosis, as well as the inflammatory disorders pulmonary arterial hypertension (PAH), idiopathic pulmonary fibrosis (IPF), and psoriasis. The FDA granted brilaroxazine orphan drug designation for the treatment of PAH and IPF.
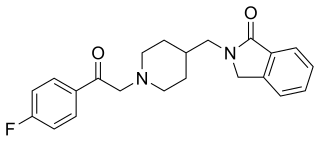
Roluperidone (former developmental code names MIN-101, CYR-101, MT-210) is a 5-HT2A and σ2 receptor antagonist under development by Minerva Neurosciences for the treatment of schizophrenia. One of its metabolites also has some affinity for the H1 receptor. Pre-clinical findings provide evidence of the effect of roluperidone on Brain-Derived Neurotrophic Factor (“BDNF”), which has been associated with neurogenesis, neuroplasticity, neuroprotection, synapse regulation, learning and memory. As of May 2018, the drug was in phase III clinical trials. In May 2020, the shares of Minerva Neurosciences plummeted 67% after the trial "failed to meet its primary endpoint of reduction in negative symptoms, and key secondary endpoints of improvement in personal and social performance measurements." However, in August 2022 Minerva submitted a New Drug Application (NDA) to the Food and Drug Administration (FDA) for the approval of roluperidone for the treatment of schizophrenia. The NDA submission in 2022 followed successful completion of a phase III clinical trial which was published in early 2022. Minerva believed that the findings of this second trial supported the claim that the drug was an effective agent for the treatment of negative symptoms in schizophrenia. However, in October 2022, FDA sent Minerva a refusal to file letter pertaining to the New Drug Application for roluperidone for treating negative symptoms in schizophrenia patients.
Aripiprazole/sertraline is a combination formulation of aripiprazole (Abilify), an atypical antipsychotic, and sertraline (Zoloft), a selective serotonin reuptake inhibitor (SSRI), which was under development by Otsuka Pharmaceutical for the treatment of major depressive disorder (MDD). It combines serotonin reuptake inhibition from sertraline and modulation of dopamine and serotonin receptors from aripiprazole. In July 2017, it was in preregistration in Japan for the treatment of MDD. However, in September 2018, the regulatory submission in Japan for MDD was withdrawn.

Dextromethorphan/bupropion (DXM/BUP), sold under the brand name Auvelity, is a combination medication for the treatment of major depressive disorder (MDD). Its active components are dextromethorphan (DXM) and bupropion. Patients who stayed on the medication had an average of 11% greater reduction in depressive symptoms than placebo in an FDA approval trial. It is taken as a tablet by mouth.
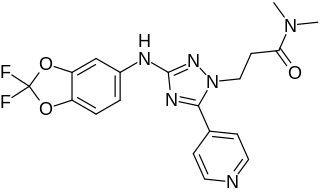
JNJ-39393406 is an experimental medication which is under development by Janssen Pharmaceutica, a division of Johnson & Johnson, for the treatment of depressive disorders and smoking withdrawal. It acts as a selective positive allosteric modulator of the α7 nicotinic acetylcholine receptor (nAChR). It does not act on the α4β2 or α3β4 nAChRs or the serotonin 5-HT3 receptor, and does not interact with a panel of 62 other receptors and enzymes. The drug has been found to lower the agonist and nicotine threshold for activation of the α7 nAChR by 10- to 20-fold and to increase the maximum agonist response of the α7 nAChR by 17- to 20-fold.

Zelquistinel is an orally active small-molecule NMDA receptor modulator which is under development for the treatment of major depressive disorder (MDD) by Gate Neurosciences, and previously by Allergan.

Hypidone (developmental code name YL-0919) is an investigational serotonergic antidepressant which is under development for the treatment of major depressive disorder. It acts as a serotonin reuptake inhibitor, 5-HT1A receptor partial agonist, and 5-HT6 receptor full agonist. It is used as the hydrochloride salt. As of January 2021, hypidone is in phase 2 clinical trials for major depressive disorder.