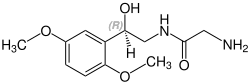| |
 Above: molecular structure of midodrine Below: 3D representation of a midodrine molecule | |
| Clinical data | |
|---|---|
| Trade names | Proamatine, others |
| Other names | ST-1085; TS-701; 3,6-Dimethoxy-β-hydroxy-N-aminoethanonyl-2-phenylethylamine; 2-Amino-N-[2-(2,5-dimethoxyphenyl)-2-hydroxyethyl]acetamide; 1-2',5'-Dimethoxyphenyl-1)-2 glycinamidoethanol |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a616030 |
| License data | |
| Pregnancy category |
|
| Routes of administration | By mouth |
| Drug class | α1-Adrenergic receptor agonist; Antihypotensive agent |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 93% (as desglymidodrine) [1] [2] |
| Metabolism | Deglycination [1] [2] |
| Metabolites | • Desglymidodrine [1] [2] |
| Onset of action | ≤1 hour [1] |
| Elimination half-life | Midodrine: 0.5 hours [2] Desglymidodrine: 2–4 hours [2] |
| Duration of action | 2–6 hours [1] [2] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.151.349 100.050.842, 100.151.349 |
| Chemical and physical data | |
| Formula | C12H18N2O4 |
| Molar mass | 254.286 g·mol−1 |
| 3D model (JSmol) | |
| Chirality | Racemic mixture |
| |
| |
Midodrine, sold under the brand name Proamatine among others, is an antihypotensive medication used to treat orthostatic hypotension (low blood pressure when standing) and urinary incontinence. [1] It is taken by mouth. [1]
Contents
- Medical uses
- Available forms
- Contraindications
- Side effects
- Pharmacokinetics
- Chemistry
- Stereochemistry
- Synthesis
- History
- Society and culture
- Names
- References
- External links
Side effects of midodrine include hypertension (high blood pressure), paresthesia, itching (pruritus), goose bumps, chills, urinary urgency, urinary retention, and urinary frequency. [1] Midodrine is a prodrug of its active metabolite desglymidodrine. [1] This metabolite acts as a selective agonist of the α1-adrenergic receptor. [1] This in turn results in vasoconstriction and increased blood pressure. [1]
Midodrine was discovered by 1971 [3] and was introduced for medical use in the United States in 1996. [4]