
PiHKAL: A Chemical Love Story is a book by Dr. Alexander Shulgin and Ann Shulgin, published in 1991. The subject of the work is psychoactive phenethylamine chemical derivatives, notably those that act as psychedelics and/or empathogen-entactogens. The main title, PiHKAL, is an acronym that stands for "Phenethylamines I Have Known and Loved."

3,4-Methylenedioxyamphetamine is an empathogen-entactogen, psychostimulant, and psychedelic drug of the amphetamine family that is encountered mainly as a recreational drug. In its pharmacology, MDA is a serotonin–norepinephrine–dopamine releasing agent (SNDRA). In most countries, the drug is a controlled substance and its possession and sale are illegal.

Methylone, also known as 3,4-methylenedioxy-N-methylcathinone (MDMC), is an empathogen and stimulant psychoactive drug. It is a member of the amphetamine, cathinone and methylenedioxyphenethylamine classes.

The substituted methylenedioxyphenethylamines represent a diverse chemical class of compounds derived from phenethylamines. This category encompasses numerous psychoactive substances with entactogenic, psychedelic, and/or stimulant properties, in addition to entheogens. These compounds find application as research chemicals, designer drugs, and recreational substances.

3,4-Methylenedioxy-N,N-dimethylamphetamine (MDDM) is a lesser-known research chemical. It is also the N,N-dimethyl analog of 3,4-methylenedioxyamphetamine (MDA). MDDM was first synthesized by Alexander Shulgin. In his book PiHKAL , the dosage is unspecified and the duration unknown. MDDM produces only mild effects that are not well characterized in PiHKAL. Very little data exists about the pharmacological properties, metabolism, and toxicity of MDDM. This compound is however occasionally encountered as an impurity in 3,4-methylenedioxy-N-methylamphetamine (MDMA) which has been synthesized by methylation of MDA using methylating reagents such as methyl iodide. An excess of reagent or a reaction temperature that is too high results in some double methylation of the amine nitrogen, yielding MDDM as well as MDMA. The presence of MDDM as an impurity can thus reveal which synthetic route was used to manufacture seized samples of MDMA.

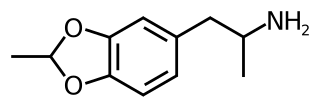
5-Methyl-3,4-methylenedioxyamphetamine (5-Methyl-MDA) is an entactogen and psychedelic designer drug of the amphetamine class. It is a ring-methylated homologue of MDA and a structural isomer of MDMA.

5-(2-Aminopropyl)-2,3-dihydrobenzofuran is a putative entactogen drug of the phenethylamine and amphetamine classes. It is an analogue of MDA where the heterocyclic 3-position oxygen from the 3,4-methylenedioxy ring has been replaced by a methylene bridge. 6-APDB is an analogue of 5-APDB where the 4-position oxygen has been replaced by a methylene bridge instead. 5-APDB was developed by a team led by David E. Nichols at Purdue University as part of their research into non-neurotoxic analogues of MDMA.

MDAI (5,6-methylenedioxy-2-aminoindane) is a drug developed in the 1990s by a team led by David E. Nichols at Purdue University. It acts as a non-neurotoxic and highly selective serotonin releasing agent (SSRA) in vitro and produces entactogen effects in humans.
A serotonin releasing agent (SRA) is a type of drug that induces the release of serotonin into the neuronal synaptic cleft. A selective serotonin releasing agent (SSRA) is an SRA with less significant or no efficacy in producing neurotransmitter efflux at other types of monoamine neurons.
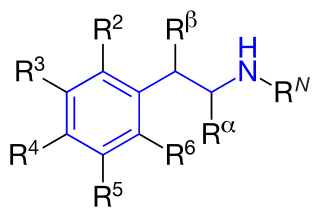
Substituted phenethylamines are a chemical class of organic compounds that are based upon the phenethylamine structure; the class is composed of all the derivative compounds of phenethylamine which can be formed by replacing, or substituting, one or more hydrogen atoms in the phenethylamine core structure with substituents.
3,4-Methylenedioxy-N-ethylamphetamine is an empathogenic psychoactive drug. MDEA is a substituted amphetamine and a substituted methylenedioxyphenethylamine. MDEA acts as a serotonin, norepinephrine, and dopamine releasing agent and reuptake inhibitor.
Substituted amphetamines are a class of compounds based upon the amphetamine structure; it includes all derivative compounds which are formed by replacing, or substituting, one or more hydrogen atoms in the amphetamine core structure with substituents. The compounds in this class span a variety of pharmacological subclasses, including stimulants, empathogens, and hallucinogens, among others. Examples of substituted amphetamines are amphetamine (itself), methamphetamine, ephedrine, cathinone, phentermine, mephentermine, tranylcypromine, bupropion, methoxyphenamine, selegiline, amfepramone (diethylpropion), pyrovalerone, MDMA (ecstasy), and DOM (STP).

6,7-Methylenedioxy-2-aminotetralin (MDAT) is a drug developed in the 1990s by a team at Purdue University led by David E. Nichols. It appears to act as a serotonin releasing agent based on rodent drug discrimination assays comparing it to MDMA, in which it fully substitutes for, and additionally lacks any kind of serotonergic neurotoxicity. Hence, MDAT is considered likely to be a non-neurotoxic, putative entactogen in humans.

6-(2-Aminopropyl)-2,3-dihydrobenzofuran is a stimulant and entactogen drug of the phenethylamine and amphetamine classes. It is an analogue of MDA where the heterocyclic 4-position oxygen from the 3,4-methylenedioxy ring has been replaced with a methylene bridge. 5-APDB (3-Desoxy-MDA) is an analogue of 6-APDB where the 3-position oxygen has been replaced with a methylene instead. 6-APDB, along with 5-APDB, was first synthesized by David E. Nichols in the early 1990s while investigating non-neurotoxic MDMA analogues.

6-Methyl-3,4-methylenedioxyamphetamine (6-Methyl-MDA) is an entactogen and psychedelic drug of the amphetamine class. It was first synthesized in the late 1990s by a team including David E. Nichols at Purdue University while investigating derivatives of 3,4-methylenedioxyamphetamine (MDA) and 3,4-methylenedioxy-N-methylamphetamine (MDMA).
3,4-Ethylidenedioxyamphetamine (EIDA) is a substituted derivative of 3,4-methylenedioxyamphetamine (MDA), which was developed by David Nichols and coworkers, in the course of research to determine the bulk tolerance around the benzodioxole portion of the MDA molecule. EIDA was found to produce similar effects to MDA in animals but with less than half the potency, while the isopropylidenedioxy derivative did not substitute for MDA and instead had sedative and convulsant effects. This shows limited bulk tolerance at this position and makes it likely the activity of EIDA will reside primarily in one enantiomer, although only the racemic mix has been studied as yet.

Difluoromethylenedioxyamphetamine is a substituted derivative of 3,4-methylenedioxyamphetamine (MDA), which was developed by Daniel Trachsel and coworkers, along with the corresponding fluorinated derivatives of MDMA, MDEA, BDB and MBDB, with the aim of finding a non-neurotoxic drug able to be used as a less harmful substitute for entactogenic drugs such as MDMA. Since a major route of the normal metabolism of these compounds is scission of the methylenedioxy ring, producing neurotoxic metabolites such as alpha-methyldopamine, it was hoped that the difluoromethylenedioxy bioisostere would show increased metabolic stability and less toxicity.

Substituted phenylmorpholines, or substituted phenmetrazines alternatively, are chemical derivatives of phenylmorpholine or of the psychostimulant drug phenmetrazine. Most such compounds act as releasers of monoamine neurotransmitters, and have stimulant effects. Some also act as agonists at serotonin receptors, and compounds with an N-propyl substitution act as dopamine receptor agonists. A number of derivatives from this class have been investigated for medical applications, such as for use as anorectics or medications for the treatment of ADHD. Some compounds have also become subject to illicit use as designer drugs.
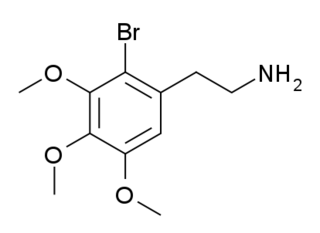
2-Bromomescaline (2-Br-M) is a derivative of the phenethylamine hallucinogen mescaline which has an unusual 2-bromo substitution. It is an agonist for serotonin receptors, with a binding affinity of 215 nM at 5-HT1A, 513 nM at 5-HT2A and 379 nM at 5-HT2C, so while it is around ten times more tightly binding than mescaline at 5-HT1A and 5-HT2A receptors, it is over twenty times more potent at 5-HT2C. It has been reported as a designer drug, first identified in Austria in January 2023.

The substituted benzofurans are a class of chemical compounds based on the heterocyclyc and polycyclic compound benzofuran. Many medicines use the benzofuran core as a scaffold, but most commonly the term is used to refer to the simpler compounds in this class which include numerous psychoactive drugs, including stimulants, psychedelics and empathogens. In general, these compounds have a benzofuran core to which a 2-aminoethyl group is attached, and combined with a range of other substituents. Some psychoactive derivatives from this family have been sold under the name Benzofury.


















