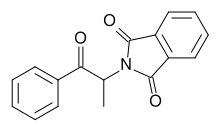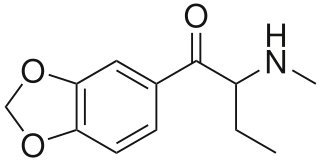
Butylone, also known as β-keto-N-methylbenzodioxolylbutanamine (βk-MBDB), is an entactogen, psychedelic, and stimulant psychoactive drug of the phenethylamine chemical class. It is the β-keto analogue of MBDB and the substituted methylenedioxyphenethylamine analogue of buphedrone.

Methylenedioxypyrovalerone (MDPV) is a stimulant of the cathinone class that acts as a norepinephrine–dopamine reuptake inhibitor (NDRI). It was first developed in the 1960s by a team at Boehringer Ingelheim. Its activity at the dopamine transporter is six times stronger than at the norepinephrine transporter and it is virtually inactive at the serotonin transporter. MDPV remained an obscure stimulant until around 2004 when it was reportedly sold as a designer drug. In the USA, products containing MDPV and labeled as bath salts were sold as recreational drugs in gas stations, similar to the marketing for Spice and K2 as incense, until it was banned in 2011.

α-Pyrrolidinopropiophenone (α-PPP), is a stimulant drug. It is similar in structure to the appetite suppressant diethylpropion and has analogous effects in animals. Little is known about this compound, but it has been detected by laboratories in Germany as an ingredient in "ecstasy" tablets seized by law enforcement authorities. This drug has been found to produce stimulant effects in animals and presumably also produces these effects in humans, based on the context in which it has been found.

Ethcathinone, also known as ethylpropion or ETH-CAT, is a stimulant drug of the phenethylamine, amphetamine, and cathinone chemical classes. It is an active metabolite of the prodrug diethylcathinone and is fully responsible for its effects. Ethcathinone has been identified as an ingredient in both quasi-legal "party pills", and, along with mephedrone, has also been reported as having been sold as "ecstasy" in the Australian city of Cairns.
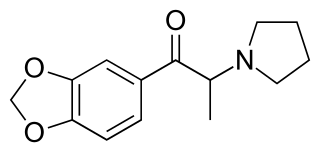
3',4'-Methylenedioxy-α-pyrrolidinopropiophenone (MDPPP) is a stimulant designer drug. It was sold in Germany in the late 1990s and early 2000s as an ingredient in imitation ecstasy (MDMA) pills. It shares a similar chemical structure with α-PPP and MDPV, and has been shown to have reinforcing effects in rats.

4'-Methyl-α-pyrrolidinopropiophenone is a stimulant drug and substituted cathinone. It is structurally very similar to α-PPP, with only one added methyl group in the para position on the phenyl ring. 4-MePPP was sold in Germany as a designer drug in the late 1990s and early 2000s, along with a number of other pyrrolidinophenone derivatives. Although it has never achieved the same international popularity as its better-known relations α-PPP and MDPV, 4-MePPP is still sometimes found as an ingredient of grey-market "bath salt" blends such as "NRG-3".

4'-Methoxy-α-pyrrolidinopropiophenone (MOPPP) is a stimulant designer drug of the pyrrolidinophenone class. It has the potential to produce euphoria, an effect shared with other classical stimulants.

Arylcyclohexylamines, also known as arylcyclohexamines or arylcyclohexanamines, are a chemical class of pharmaceutical, designer, and experimental drugs.

3',4'-Methylenedioxy-α-pyrrolidinobutyrophenone (MDPBP) is a stimulant of the cathinone class developed in the 1960s, which has been reported as a novel designer drug. MDPBP is sometimes sold under the name "NRG-1" as a mixture with other cathinone derivatives, including flephedrone, pentylone, MαPPP and its higher homologue MDPV. As with other cathinones, MDPBP has been shown to have reinforcing effects in rats.

4'-Methyl-α-pyrrolidinohexiophenone (MPHP) is a stimulant compound which has been reported as a novel designer drug. It is closely related to pyrovalerone, being simply its chain-lengthened homologue. In the pyrrolidinophenone series, stimulant activity is maintained so long as the positions of the aryl, ketone and pyrrolidinyl groups are held constant, while the alkyl backbone can be varied anywhere between three and as many as seven carbons, with highest potency usually seen with the pentyl or isohexyl backbone, and a variety of substituents are tolerated on the aromatic ring.
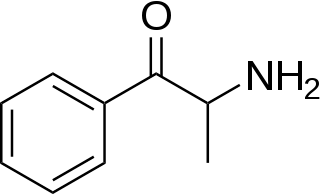
Substituted cathinones, which include some stimulants and entactogens, are derivatives of cathinone. They feature a phenethylamine core with an alkyl group attached to the alpha carbon, and a ketone group attached to the beta carbon, along with additional substitutions. Cathinone occurs naturally in the plant khat whose leaves are chewed as a recreational drug.
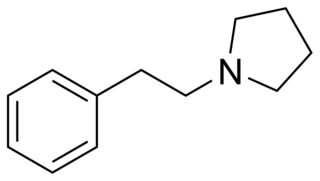
1-(2-Phenylethyl)pyrrolidine (PEP) is a chemical compound. It is an analogue of 2-phenylethylamine where the amine has been replaced by a pyrrolidine ring.

βk-2C-B is a novel psychedelic substance. It is the beta (β) ketone structural analogue of 2C-B, a psychedelic drug of the 2C family. It is used as a recreational drug, usually taken orally. βk-2C-B is a controlled substance in Canada, Germany, Switzerland, and the United Kingdom.

α-Pyrrolidinohexiophenone is a synthetic stimulant drug of the cathinone class developed in the 1960s which has been reported as a novel designer drug.
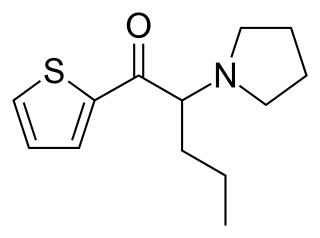
α-Pyrrolidinopentiothiophenone is a synthetic stimulant of the cathinone class that has been sold online as a designer drug. It is an analogue of α-PVP where the phenyl ring has been replaced by thiophene.
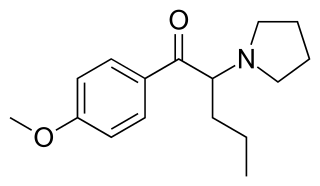
4'-Methoxy-α-pyrrolidinopentiophenone is a stimulant drug of the cathinone class that has been sold online as a designer drug.
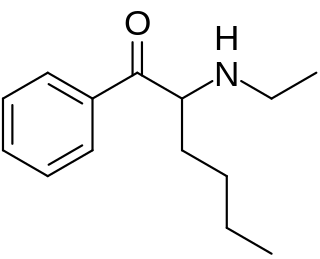
N-Ethylhexedrone (also known as α-ethylaminocaprophenone, N-ethylnorhexedrone, hexen, and NEH) is a stimulant of the cathinone class that acts as a norepinephrine–dopamine reuptake inhibitor (NDRI) with IC50 values of 0.0978 and 0.0467 μM, respectively. N-Ethylhexedrone was first mentioned in a series of patents by Boehringer Ingelheim in the 1960s which led to the development of the better-known drug methylenedioxypyrovalerone (MDPV). Since the mid-2010s, N-ethylhexedrone has been sold online as a designer drug. In 2018, N-ethylhexedrone was the second most common drug of the cathinone class to be identified in Drug Enforcement Administration seizures.

TH-PVP is a substituted cathinone derivative which has been sold as a designer drug. It was first identified by a forensic laboratory in Hungary in 2015, but has subsequently been found in numerous other countries around the world including Spain, Belgium, Poland, Turkey and Brazil. Pharmacological studies in vitro showed it to inhibit reuptake and promote the release of monoamine neurotransmitters with some selectivity for serotonin, but it failed to produce stimulant effects in animals, and has a pharmacological profile more comparable to that of sedating empathogens such as MDAI and 5-Methyl-MDA.