
Methcathinone is a monoamine alkaloid and psychoactive stimulant, a substituted cathinone. It is used as a recreational drug due to its potent stimulant and euphoric effects and is considered to be addictive, with both physical and psychological withdrawal occurring if its use is discontinued after prolonged or high-dosage administration. It is usually snorted, but can be smoked, injected, or taken orally.
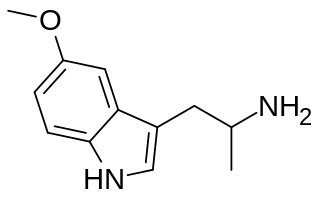
5-MeO-αMT, or 5-methoxy-α-methyltryptamine, also known as α,O-dimethylserotonin (Alpha-O), is a serotonergic psychedelic of the tryptamine family. It is a derivative of α-methyltryptamine (αMT) and an analogue of 5-MeO-DMT.
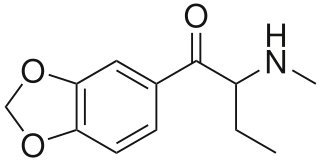
Butylone, also known as β-keto-N-methylbenzodioxolylbutanamine (βk-MBDB), is an entactogen, psychedelic, and stimulant psychoactive drug of the phenethylamine, amphetamine, phenylisobutylamine, and cathinone families. It is the β-keto analogue of MBDB and the substituted methylenedioxyphenethylamine analogue of buphedrone.
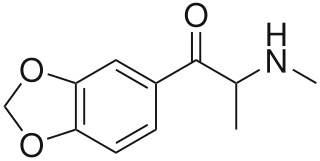
Methylone, also known as 3,4-methylenedioxy-N-methylcathinone (MDMC), is an empathogen and stimulant psychoactive drug. It is a member of the amphetamine, cathinone and methylenedioxyphenethylamine classes.

Methylenedioxypyrovalerone is a stimulant of the cathinone class that acts as a norepinephrine–dopamine reuptake inhibitor (NDRI). It was first developed in the 1960s by a team at Boehringer Ingelheim. Its activity at the dopamine transporter is six times stronger than at the norepinephrine transporter and it is virtually inactive at the serotonin transporter. MDPV remained an obscure stimulant until around 2004 when it was reportedly sold as a designer drug. In the US, products containing MDPV and labeled as bath salts were sold as recreational drugs in gas stations, similar to the marketing for Spice and K2 as incense, until it was banned in 2011.

α-Pyrrolidinopropiophenone (α-PPP), is a stimulant drug. It is similar in structure to the appetite suppressant diethylpropion and has analogous effects in animals. Little is known about this compound, but it has been detected by laboratories in Germany as an ingredient in "ecstasy" tablets seized by law enforcement authorities. This drug has been found to produce stimulant effects in animals and produces highly stimulating effects in humans, based on the experiences of the individuals who have tried it. Most of the individuals who have tried it prefer α-PVP to it, but prefer this drug over α-PVT. It is said to lack euphoria compared to α-PVP.

4'-Methyl-α-pyrrolidinopropiophenone is a stimulant drug and substituted cathinone. It is structurally very similar to α-PPP, with only one added methyl group in the para position on the phenyl ring. 4-MePPP was sold in Germany as a designer drug in the late 1990s and early 2000s, along with a number of other pyrrolidinophenone derivatives. Although it has never achieved the same international popularity as its better-known relations α-PPP and MDPV, 4-MePPP is still sometimes found as an ingredient of grey-market "bath salt" blends such as "NRG-3".

3',4'-Methylenedioxy-α-pyrrolidinobutyrophenone (MDPBP) is a stimulant of the cathinone class developed in the 1960s, which has been reported as a novel designer drug. MDPBP is sometimes sold under the name "NRG-1" as a mixture with other cathinone derivatives, including flephedrone, pentylone, MαPPP and its higher homologue MDPV. As with other cathinones, MDPBP has been shown to have reinforcing effects in rats.

α-Pyrrolidinopentiophenone (α-PVP), also known as α-pyrrolidinovalerophenone, O-2387, β-keto-prolintane, prolintanone, or desmethylpyrovalerone, is a synthetic stimulant of the cathinone class developed in the 1960s that has been sold as a designer drug and often consumed for recreational reasons. α-PVP is chemically related to pyrovalerone and is the ketone analog of prolintane.

Naphyrone, also known as O-2482 and naphthylpyrovalerone, is a substituted cathinone drug derived from pyrovalerone that acts as a serotonin–norepinephrine–dopamine reuptake inhibitor (SNDRI), producing stimulant effects and has been reported as a novel designer drug. No safety or toxicity data is available on the drug.

Substituted cathinones, or simply cathinones, which include some stimulants and entactogens, are derivatives of cathinone. They feature a phenethylamine core with an alkyl group attached to the alpha carbon, and a ketone group attached to the beta carbon, along with additional substitutions. Cathinone occurs naturally in the plant khat whose leaves are chewed as a recreational drug.
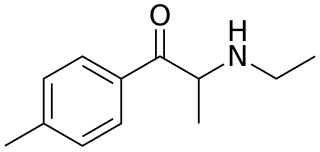
4-Methylethcathinone or 4-MEC is a chemical that bears a chemical resemblance to mephedrone. Due to its similarity to mephedrone, it is thought to be a stimulant and entactogen drug of the phenethylamine, amphetamine, and cathinone chemical classes. It has been marketed alone or in mixtures with other substituted cathinones under the name "NRG-2", although other blends such as "NRG-1" may have been more ambiguous with their ingredients.

Pentedrone is a stimulant of the cathinone class that has been sold as a designer drug and has been found since 2010 as an ingredient in a number of "bath salt" mixes sold as legal highs.

α-Pyrrolidinohexiophenone is a synthetic stimulant drug of the cathinone class developed in the 1960s which has been reported as a novel designer drug.

4-Chloromethcathinone is a stimulant drug of the cathinone class that has been sold online as a designer drug.
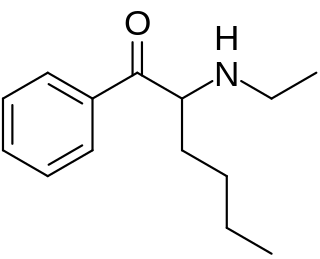
N-Ethylhexedrone (also known as α-ethylaminocaprophenone, N-ethylnorhexedrone, hexen, and NEH) is a stimulant of the cathinone class that acts as a norepinephrine–dopamine reuptake inhibitor (NDRI) with IC50 values of 0.0978 and 0.0467 μM, respectively. N-Ethylhexedrone was first mentioned in a series of patents by Boehringer Ingelheim in the 1960s which led to the development of the better-known drug methylenedioxypyrovalerone (MDPV). Since the mid-2010s, N-ethylhexedrone has been sold online as a designer drug. In 2018, N-ethylhexedrone was the second most common drug of the cathinone class to be identified in Drug Enforcement Administration seizures.
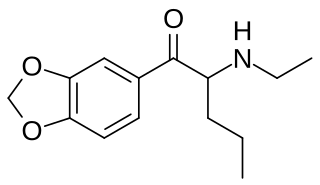
N-Ethylpentylone is a substituted cathinone and stimulant drug which was developed in the 1960s.

N-Ethylpentedrone is a chemical compound of the substituted cathinone class. Since the mid-2010s, NEP has been sold online as a designer drug. It is the N-ethyl analog of pentedrone.

TH-PVP is a substituted cathinone derivative which has been sold as a designer drug. It was first identified by a forensic laboratory in Hungary in 2015, but has subsequently been found in numerous other countries around the world including Spain, Belgium, Poland, Turkey and Brazil.
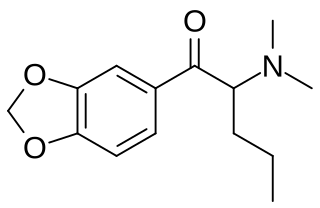
N,N-Dimethylpentylone is a substituted cathinone derivative with stimulant effects, which has been sold as a designer drug, first detected in Sweden in 2014.




















