
5-HT receptors, 5-hydroxytryptamine receptors, or serotonin receptors, are a group of G protein-coupled receptor and ligand-gated ion channels found in the central and peripheral nervous systems. They mediate both excitatory and inhibitory neurotransmission. The serotonin receptors are activated by the neurotransmitter serotonin, which acts as their natural ligand.

Agomelatine, sold under the brand names Valdoxan and Thymanax, among others, is an atypical antidepressant most commonly used to treat major depressive disorder and generalized anxiety disorder. One review found that it is as effective as other antidepressants with similar discontinuation rates overall but fewer discontinuations due to side effects. Another review also found it was similarly effective to many other antidepressants.
The 5-HT2C receptor is a subtype of the 5-HT2 receptor that binds the endogenous neurotransmitter serotonin (5-hydroxytryptamine, 5-HT). Like all 5-HT2 receptors, it is a G protein-coupled receptor (GPCR) that is coupled to Gq/G11 and mediates excitatory neurotransmission. HTR2C denotes the human gene encoding for the receptor, that in humans is located on the X chromosome. As males have one copy of the gene and females have one of the two copies of the gene repressed, polymorphisms at this receptor can affect the two sexes to differing extent.

The serotonin 1A receptor is a subtype of serotonin receptors, or 5-HT receptors, that binds serotonin, also known as 5-HT, a neurotransmitter. 5-HT1A is expressed in the brain, spleen, and neonatal kidney. It is a G protein-coupled receptor (GPCR), coupled to the Gi protein, and its activation in the brain mediates hyperpolarization and reduction of firing rate of the postsynaptic neuron. In humans, the serotonin 1A receptor is encoded by the HTR1A gene.
5-Hydroxytryptamine receptor 2B (5-HT2B) also known as serotonin receptor 2B is a protein that in humans is encoded by the HTR2B gene. 5-HT2B is a member of the 5-HT2 receptor family that binds the neurotransmitter serotonin (5-hydroxytryptamine, 5-HT). Like all 5-HT2 receptors, the 5-HT2B receptor is Gq/G11-protein coupled, leading to downstream activation of phospholipase C.

The 5HT6 receptor is a subtype of 5HT receptor that binds the endogenous neurotransmitter serotonin (5-hydroxytryptamine, 5HT). It is a G protein-coupled receptor (GPCR) that is coupled to Gs and mediates excitatory neurotransmission. HTR6 denotes the human gene encoding for the receptor.
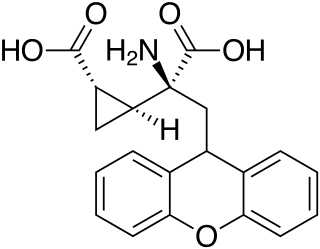
LY-341495 is a research drug developed by the pharmaceutical company Eli Lilly, which acts as a potent and selective orthosteric antagonist for the group II metabotropic glutamate receptors (mGluR2/3).

RS-102221 is a drug developed by Hoffmann–La Roche, which was one of the first compounds discovered that acts as a potent and selective antagonist at the serotonin 5-HT2C receptor, with around 100× selectivity over the closely related 5-HT2A and 5-HT2B receptors. It has anxiolytic effects in animal studies, increases the effectiveness of SSRI antidepressants, and shows a complex interaction with cocaine, increasing some effects but decreasing others, reflecting a role for the 5-HT2C receptor in regulation of the dopamine signalling system in the brain.

BW-723C86 is a tryptamine derivative drug which acts as a 5-HT2B receptor agonist. It has anxiolytic effects in animal studies, and is also used for investigating the function of the 5-HT2B receptor in a range of other tissues.

A serotonin releasing agent (SRA) is a type of drug that induces the release of serotonin into the neuronal synaptic cleft. A selective serotonin releasing agent (SSRA) is an SRA with less significant or no efficacy in producing neurotransmitter efflux at other types of monoamine neurons, including dopamine and norepinephrine neurons.
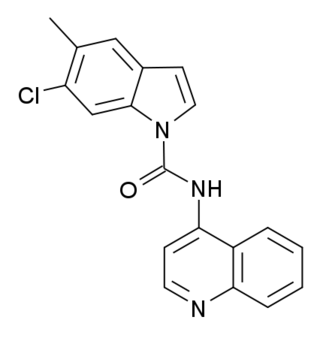
SB-215505 is a drug which acts as a potent and selective antagonist at the serotonin 5-HT2B receptor, with good selectivity over the related 5-HT2A and 5-HT2C receptors. It is used in scientific research into the function of the 5-HT2 family of receptors, especially to study the role of 5-HT2B receptors in the heart, and to distinguish 5-HT2B-mediated responses from those produced by 5-HT2A or 5-HT2C.

CP-809101 is a drug which acts as a potent and selective 5-HT2C receptor agonist. It had promising results in animal models of obesity and psychosis, but associated with genotoxicity which means that future use will be restricted to scientific research applications only.

Pruvanserin is a selective 5-HT2A receptor antagonist which was under development by Eli Lilly and Company for the treatment of insomnia. It was in phase II clinical trials in 2008 but appears to have been discontinued as it is no longer in the company's development pipeline. In addition to its sleep-improving properties, pruvanserin has also been shown to have antidepressant, anxiolytic, and working memory-enhancing effects in animal studies.
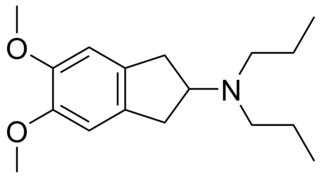
PNU-99,194(A) (or U-99,194(A)) is a drug which acts as a moderately selective D3 receptor antagonist with ~15-30-fold preference for D3 over the D2 subtype. Though it has substantially greater preference for D3 over D2, the latter receptor does still play some role in its effects, as evidenced by the fact that PNU-99,194 weakly stimulates both prolactin secretion and striatal dopamine synthesis, actions it does not share with the more selective (100-fold) D3 receptor antagonists S-14,297 and GR-103,691.

L-741,626 is a drug which acts as a potent and selective antagonist for the dopamine receptor D2. It has good selectivity over the related D3 and D4 subtypes and other receptors. L-741,626 is used for laboratory research into brain function and has proved particularly useful for distinguishing D2 mediated responses from those produced by the closely related D3 subtype, and for studying the roles of these subtypes in the action of cocaine and amphetamines in the brain.

SB-206553 is a drug which acts as a mixed antagonist for the 5-HT2B and 5-HT2C serotonin receptors.

SB-243213 is a research chemical which acts as a selective inverse agonist for the 5HT2C receptor and has anxiolytic effects. It has better than 100x selectivity for 5-HT2C over all other receptor subtypes tested, and a longer duration of action compared to older 5-HT2C antagonist ligands.

Locomotor activity is a measure of animal behavior which is employed in scientific research.

A motivation-enhancing drug, also known as a pro-motivational drug, is a drug which increases motivation. Drugs enhancing motivation can be used in the treatment of motivational deficits, for instance in depression, schizophrenia, and attention deficit hyperactivity disorder (ADHD). They can also be used in the treatment of disorders of diminished motivation (DDMs), including apathy, abulia, and akinetic mutism, disorders that can be caused by conditions like stroke, traumatic brain injury (TBI), and neurodegenerative diseases. Motivation-enhancing drugs are used non-medically by healthy people to increase motivation and productivity as well, for instance in educational contexts.

SB-221284 is a selective serotonin 5-HT2C and 5-HT2B receptor antagonist which is used in scientific research.




















