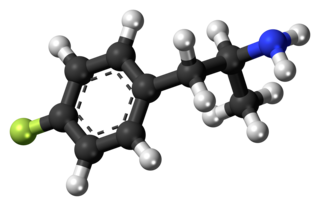
4-Fluoroamphetamine, also known as para-fluoroamphetamine (PFA) is a psychoactive research chemical of the phenethylamine and substituted amphetamine chemical classes. It produces stimulant and entactogenic effects. As a recreational drug, 4-FA is sometimes sold along with related compounds such as 2-fluoroamphetamine and 4-fluoromethamphetamine.

Chlorphentermine, sold under the brand names Apsedon, Desopimon, and Lucofen, is a serotonergic appetite suppressant of the amphetamine family. Developed in 1962, it is the para-chloro derivative of the better-known appetite suppressant phentermine, which is still in current use.

1-Phenyl-2-propylaminopentane is an experimental drug related to selegiline which acts as a catecholaminergic activity enhancer (CAE).

para-Chloroamphetamine (PCA), also known as 4-chloroamphetamine (4-CA), is a serotonin–norepinephrine–dopamine releasing agent (SNDRA) and serotonergic neurotoxin of the amphetamine family. It is used in scientific research in the study of the serotonin system, as a serotonin releasing agent (SRA) at lower doses to produce serotonergic effects, and as a serotonergic neurotoxin at higher doses to produce long-lasting depletions of serotonin.
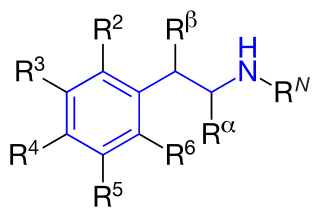
Substituted phenethylamines are a chemical class of organic compounds that are based upon the phenethylamine structure; the class is composed of all the derivative compounds of phenethylamine which can be formed by replacing, or substituting, one or more hydrogen atoms in the phenethylamine core structure with substituents. Phenylethylamines are also generally found to be central nervous system stimulants with many also being entactogens/empathogens, and hallucinogens.

para-Bromoamphetamine (PBA), also known as 4-bromoamphetamine (4-BA), is an amphetamine derivative which acts as a serotonin-norepinephrine-dopamine releasing agent (SNDRA) and produces stimulant effects.

3-Fluoroamphetamine is a stimulant drug from the amphetamine family which acts as a monoamine releaser with similar potency to methamphetamine but more selectivity for dopamine and norepinephrine release over serotonin. It is self-administered by mice to a similar extent to related drugs such as 4-fluoroamphetamine and 3-methylamphetamine.
Substituted amphetamines, or simply amphetamines, are a class of compounds based upon the amphetamine structure; it includes all derivative compounds which are formed by replacing, or substituting, one or more hydrogen atoms in the amphetamine core structure with substituents. The compounds in this class span a variety of pharmacological subclasses, including stimulants, empathogens, and hallucinogens, among others. Examples of substituted amphetamines are amphetamine (itself), methamphetamine, ephedrine, cathinone, phentermine, mephentermine, tranylcypromine, bupropion, methoxyphenamine, selegiline, amfepramone (diethylpropion), pyrovalerone, MDMA (ecstasy), and DOM (STP).
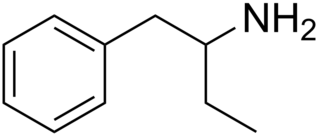
Phenylisobutylamine, also known as α-ethylphenethylamine, Butanphenamine, B or AEPEA, is a stimulant drug of the phenethylamine class. It is a higher homologue of amphetamine, differing from amphetamine's molecular structure only by the substitution of the methyl group at the alpha position of the side chain with an ethyl group.

1-Phenylpiperazine is a simple chemical compound and drug featuring a phenyl group bound to a piperazine ring. The suffix ‘-piprazole’ is sometimes used in the names of drugs to indicate they belong to this class.

Thiopropamine is a stimulant drug which is an analogue of amphetamine where the phenyl ring has been replaced by thiophene. It has similar stimulant effects to amphetamine but with around one third the potency. The N-methyl and thiophen-3-yl analogues are also known and are somewhat more potent, though still generally weaker than the corresponding amphetamines.
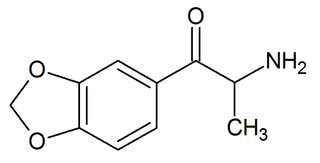
3,4-Methylenedioxycathinone is an empathogen and stimulant of the phenethylamine, amphetamine, and cathinone classes and the β-keto analogue of MDA.
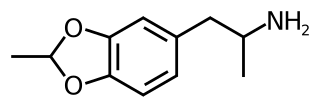
3,4-Ethylidenedioxyamphetamine (EIDA) is a substituted derivative of 3,4-methylenedioxyamphetamine (MDA), which was developed by David Nichols and coworkers, in the course of research to determine the bulk tolerance around the benzodioxole portion of the MDA molecule. EIDA was found to produce similar effects to MDA in animals but with less than half the potency, while the isopropylidenedioxy derivative did not substitute for MDA and instead had sedative and convulsant effects. This shows limited bulk tolerance at this position and makes it likely the activity of EIDA will reside primarily in one enantiomer, although only the racemic mix has been studied as yet.

Substituted tryptamines, or simply tryptamines, also known as serotonin analogues (i.e., 5-hydroxytryptamine analogues), are organic compounds which may be thought of as being derived from tryptamine itself. The molecular structures of all tryptamines contain an indole ring, joined to an amino (NH2) group via an ethyl (−CH2–CH2−) sidechain. In substituted tryptamines, the indole ring, sidechain, and/or amino group are modified by substituting another group for one of the hydrogen (H) atoms.

3-Fluoromethamphetamine (3-FMA) is a stimulant drug related to methamphetamine and 3-fluoroamphetamine. It has been sold online as a designer drug.

5-Chloro-α-methyltryptamine (5-Chloro-αMT), also known as PAL-542, is a tryptamine derivative related to α-methyltryptamine (αMT) and one of only a few known specific serotonin-dopamine releasing agents (SDRAs). It has been investigated in animals as a potential treatment for cocaine dependence. The EC50 values of 5-chloro-αMT in evoking the in vitro release of serotonin (5-HT), dopamine (DA), and norepinephrine (NE) in rat synaptosomes were reported as 16 nM, 54 nM, and 3434 nM, with an NE/DA ratio of 63.6 and a DA/5-HT ratio of 3.38, indicating that it is a highly specific and well-balanced SDRA. However, 5-chloro-αMT has also been found to act as a potent full agonist of the 5-HT2A receptor, with an EC50 value of 6.27 nM and an efficacy of 105%. It is likely to act as a potent agonist of other serotonin receptors as well.

para-Chloromethamphetamine is a stimulant that is the N-methyl derivative and prodrug of the neurotoxic drug para-chloroamphetamine (4-CA). It has been found to decrease serotonin in rats. Further investigation into the long-term effects of chloroamphetamines discovered that administration of 4-CMA caused a prolonged reduction in the levels of serotonin and the activity of tryptophan hydroxylase in the brain one month after injection of a single dose of the drug.

The substituted benzofurans are a class of chemical compounds based on the heterocyclyc and polycyclic compound benzofuran. Many medicines use the benzofuran core as a scaffold, but most commonly the term is used to refer to the simpler compounds in this class which include numerous psychoactive drugs, including stimulants, psychedelics and empathogens. In general, these compounds have a benzofuran core to which a 2-aminoethyl group is attached, and combined with a range of other substituents. Some psychoactive derivatives from this family have been sold under the name Benzofury.

5-Methylmethiopropamine is a stimulant drug which is a ring-substituted derivative of methiopropamine. It is not a substituted cathinone derivative like mephedrone, as it lacks a ketone group at the β position of the aliphatic side chain, but instead more closely resembles substituted amphetamines. It has been sold as a designer drug, first being identified in Germany in June 2020.

3-Chloroamphetamine (3-CA), also known as meta-chloroamphetamine, is a psychostimulant of the amphetamine family and a potent serotonergic neurotoxin related to para-chloroamphetamine (PCA). It is a potent serotonin–norepinephrine–dopamine releasing agent (SNDRA).



















