
Phenmetrazine, sold under the brand name Preludin among others, is a stimulant drug first synthesized in 1952 and originally used as an appetite suppressant, but withdrawn from the market in the 1980s due to widespread misuse. It was initially replaced by its analogue phendimetrazine which functions as a prodrug to phenmetrazine, but now it is rarely prescribed, due to concerns of misuse and addiction. Chemically, phenmetrazine is a substituted amphetamine containing a morpholine ring or a substituted phenylmorpholine.

4-Methylaminorex is a stimulant drug of the 2-amino-5-aryloxazoline group that was first synthesized in 1960 by McNeil Laboratories. It is also known by its street name "U4Euh" ("Euphoria"). It is banned in many countries as a stimulant. 4-Methylaminorex has effects comparable to methamphetamine but with a longer duration.

Aminorex, sold under the brand names Menocil and Apiquel among others, is a weight loss (anorectic) stimulant drug. It was withdrawn from the market after it was found to cause pulmonary hypertension (PPH). In the United States, aminorex is a Schedule I controlled substance.

Etilamfetamine, also known as N-ethylamphetamine and formerly sold under the brand names Apetinil and Adiparthrol, is a stimulant drug of the amphetamine family. It was invented in the early 20th century and was subsequently used as an anorectic or appetite suppressant in the 1950s, but was not as commonly used as other amphetamines such as amphetamine, methamphetamine, and benzphetamine, and was largely discontinued once newer drugs such as phenmetrazine were introduced.

Propylamphetamine is a psychostimulant of the amphetamine family which was never marketed. It was first developed in the 1970s, mainly for research into the metabolism of, and as a comparison tool to, other amphetamines.

Naphthylaminopropane, also known as naphthylisopropylamine (NIPA), is an experimental drug that was under investigation for the treatment of alcohol and stimulant addiction.

Norfenfluramine, or 3-trifluoromethylamphetamine, is a never-marketed drug of the amphetamine family and a major active metabolite of the appetite suppressants fenfluramine and benfluorex. The compound is a racemic mixture of two enantiomers with differing activities, dexnorfenfluramine and levonorfenfluramine.
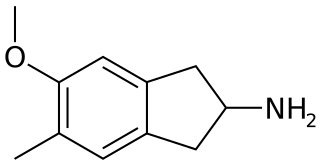
5-Methoxy-6-methyl-2-aminoindane (MMAI) is a drug of the 2-aminoindane group developed in the 1990s by a team led by David E. Nichols at Purdue University. It acts as a less neurotoxic and highly selective serotonin releasing agent (SSRA) and produces entactogenic effects in humans. It has been sold as a designer drug and research chemical online since 2010.

2-Aminoindane (2-AI) is an aminoindane and research chemical with applications in neurologic disorders and psychotherapy that has also been sold as a designer drug. It acts as a selective substrate for NET and DAT.

A dopamine releasing agent (DRA) is a type of drug which induces the release of dopamine in the body and/or brain.

4-Methylamphetamine (4-MA), also known by the former proposed brand name Aptrol, is a stimulant and anorectic drug of the amphetamine family. It is structurally related to mephedrone (4-methylmethcathinone).

4-Methylmethamphetamine (4-MMA), also known as mephedrine, is a putative stimulant and entactogen drug of the amphetamine family. It acts as a serotonin–norepinephrine–dopamine releasing agent (SNDRA). The drug is the β-deketo analogue of mephedrone and the N-methyl analogue of 4-methylamphetamine (4-MA).

Pseudophenmetrazine is a psychostimulant of the phenylmorpholine group. It is the N-demethylated and cis-configured analogue of phendimetrazine as well as the cis-configured stereoisomer of phenmetrazine. In addition, along with phenmetrazine, it is believed to be one of the active metabolites of phendimetrazine, which itself is inactive and behaves merely as a prodrug.

4,4'-Dimethylaminorex, sometimes referred to by the street name "Serotoni", is a psychostimulant and entactogen designer drug related to aminorex, 4-methylaminorex, and pemoline. It was first detected in the Netherlands in December 2012, and has been sold as a designer drug around Europe since mid-2013.

Methamnetamine is a triple monoamine releasing agent and N-methyl analog of the non-neurotoxic experimental drug naphthylaminopropane and the naphthalene analog of methamphetamine. It has been sold online as a designer drug.

BMAPN, also known as βk-methamnetamine or as 2-naphthylmethcathinone, is a substituted cathinone derivative with stimulant effects. It inhibits dopamine reuptake and has rewarding and reinforcing properties in animal studies. It is banned under drug analogue legislation in a number of jurisdictions. The drug was at one point marketed under the name NRG-3, although only a minority of samples of substances sold under this name have been found to actually contain BMAPN, with most such samples containing mixtures of other cathinone derivatives.
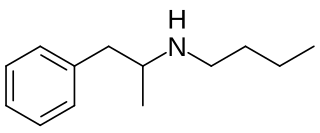
Butylamphetamine is a psychostimulant of the substituted amphetamine family which was never marketed.

2-Phenylmorpholine is the parent compound of the substituted phenylmorpholine class of compounds. Examples of 2-phenylmorpholine derivatives include phenmetrazine (3-methyl-2-phenylmorpholine), phendimetrazine ( -3,4-dimethyl-2-phenylmorpholine), and pseudophenmetrazine ( -3-methyl-2-phenylmorpholine), which are monoamine releasing agents (MRAs) and psychostimulants. 2-Phenylmorpholine itself is a potent norepinephrine–dopamine releasing agent (NDRA) and hence may act as a stimulant similarly.

Ethylnaphthylaminopropane is a monoamine releasing agent (MRA) of the amphetamine family that is related to naphthylaminopropane and methamnetamine. It acts specifically as a serotonin–norepinephrine–dopamine releasing agent (SNDRA). However, ENAP is unusual in being a partial releaser of serotonin and dopamine and a full releaser of norepinephrine.

Naphthylmetrazine, also known as 3-methyl-2-(2′-naphthyl)morpholine, is a monoamine releasing agent (MRA) and monoamine reuptake inhibitor (MRI) of the phenylmorpholine family related to phenmetrazine. It is the analogue of phenmetrazine in which the phenyl ring has been replaced with a naphthalene ring.




















