
4-Methylaminorex is a stimulant drug of the 2-amino-5-aryloxazoline group that was first synthesized in 1960 by McNeil Laboratories. It is also known by its street name "U4Euh" ("Euphoria"). It is banned in many countries as a stimulant. 4-Methylaminorex has effects comparable to methamphetamine but with a longer duration.

Aminorex, sold under the brand names Menocil and Apiquel among others, is a weight loss (anorectic) stimulant drug. It was withdrawn from the market after it was found to cause pulmonary hypertension (PPH). In the United States, aminorex is a Schedule I controlled substance.

Levamisole, sold under the brand name Ergamisol among others, is a medication used to treat parasitic worm infections, specifically ascariasis and hookworm infections. It is taken by mouth.
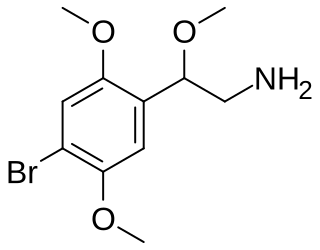
BOB (4-bromo-2,5,beta-trimethoxyphenethylamine) is a lesser-known psychedelic drug. It is the beta-methoxy analog of 2C-B. BOB was first synthesized by Alexander Shulgin. In his book PiHKAL, the dosage range is listed as 10–20 mg, and the duration listed as 10–20 hours. BOB produces an altered state of consciousness, tinnitus, a pleasant tingling throughout the body, and a sense of awareness. Very little data exists about the pharmacological properties, metabolism, and toxicity of BOB.

Fenozolone (Ordinator) was developed by Laboratoires Dausse in the 1960s and is a psychostimulant related to pemoline.

Fluminorex is a centrally acting sympathomimetic which is related to other drugs such as aminorex and pemoline. It was developed as an appetite suppressant by McNeil Laboratories in the 1950s.

Clominorex is a centrally acting sympathomimetic which is related to other drugs such as aminorex and pemoline. It was developed as an appetite suppressant by McNeil Laboratories in the 1950s.

Cyclazodone is a centrally acting stimulant drug developed by American Cyanamid Company in the 1960s. The drug is related to other drugs such as pemoline and thozalinone. It displayed a favorable therapeutic index and margin of safety in comparison to pemoline and other N-lower-alkyl-substituted pemoline derivatives. The patents concluded that cyclazodone possessed properties efficacious in reducing fatigue and as a potential anorectic. Structural congeners of pemoline have been described as "excitants with unique properties distinguishing them from the sympathomimetic amines" whilst displaying less stimulatory activity and toxicity compared to amphetamine.

A dopamine releasing agent (DRA) is a type of drug which induces the release of dopamine in the body and/or brain.
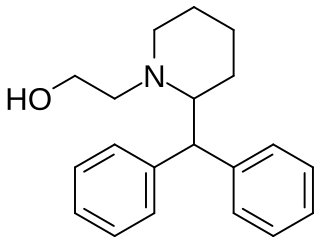
Difemetorex is a stimulant drug of the piperidine class which was used as an appetite suppressant, but produced intolerable side effects such as insomnia which limited its clinical use. It was introduced in France by Ciba-Geigy in 1966 but is now no longer marketed.

4,4'-Dimethylaminorex, sometimes referred to by the street name "Serotoni", is a psychostimulant and entactogen designer drug related to aminorex, 4-methylaminorex, and pemoline. It was first detected in the Netherlands in December 2012, and has been sold as a designer drug around Europe since mid-2013.

Substituted phenylmorpholines, or substituted phenmetrazines alternatively, are chemical derivatives of 2-phenylmorpholine or of the psychostimulant drug phenmetrazine. Most such compounds act as releasers of monoamine neurotransmitters, and have stimulant effects. Some also act as agonists at serotonin receptors, and compounds with an N-propyl substitution act as dopamine receptor agonists. A number of derivatives from this class have been investigated for medical applications, such as for use as anorectics or medications for the treatment of ADHD. Some compounds have also become subject to illicit use as designer drugs.

2,5-dimethoxy-4-bromophenylpiperazine (2C-B-PP) is a drug of the phenylpiperazine class. It acts as an agonist at serotonin receptors, and in studies on rats substituted for the psychedelic amphetamine derivative DOM with around 1/10 the potency but similar rates of stimulus-appropriate responding at the highest dose.

2C-B-aminorex (2C-B-AR) is a recreational designer drug with psychedelic effects. It is a substituted aminorex derivative which was first identified in Sweden in June 2019. Structurally, it is a hybrid of 4-bromo-2,5-dimethoxyphenethylamine (2C-B) and aminorex.
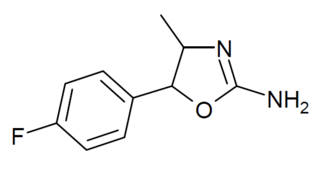
4'-Fluoro-4-methylaminorex is a recreational designer drug from the substituted aminorex family, with stimulant effects. It was first detected in Slovenia in 2018. It was made illegal in Italy in March 2020.

3',4'-Methylenedioxy-4-methylaminorex (MDMAR) is a recreational designer drug from the substituted aminorex family, with monoamine-releasing effects. It is a potent serotonin–norepinephrine–dopamine releasing agent (SNDRA).

3-Chlorophenmetrazine is a recreational designer drug with stimulant effects. It is a substituted phenylmorpholine derivative, closely related to better known drugs such as phenmetrazine and 3-fluorophenmetrazine.
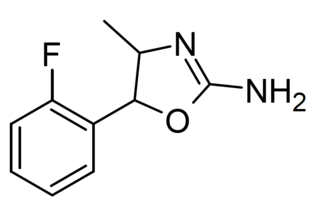
2'-Fluoro-4-methylaminorex is a recreational designer drug from the substituted aminorex family, with stimulant effects, first reported in 2018.

4'-Bromo-4-methylaminorex is a designer drug from the substituted aminorex family, first definitively identified in Austria in January 2022. Its pharmacological activity has not been reported, but it is believed to have stimulant effects.



















