
Monoamine oxidases (MAO) are a family of enzymes that catalyze the oxidation of monoamines, employing oxygen to clip off their amine group. They are found bound to the outer membrane of mitochondria in most cell types of the body. The first such enzyme was discovered in 1928 by Mary Bernheim in the liver and was named tyramine oxidase. The MAOs belong to the protein family of flavin-containing amine oxidoreductases.

A catecholamine is a monoamine neurotransmitter, an organic compound that has a catechol and a side-chain amine.

Phenelzine, sold under the brand name Nardil among others, is a non-selective and irreversible monoamine oxidase inhibitor (MAOI) of the hydrazine family which is primarily used as an antidepressant and anxiolytic to treat depression and anxiety. Along with tranylcypromine and isocarboxazid, phenelzine is one of the few non-selective and irreversible MAOIs still in widespread clinical use.

Phenethylamine (PEA) is an organic compound, natural monoamine alkaloid, and trace amine, which acts as a central nervous system stimulant in humans. In the brain, phenethylamine regulates monoamine neurotransmission by binding to trace amine-associated receptor 1 (TAAR1) and inhibiting vesicular monoamine transporter 2 (VMAT2) in monoamine neurons. To a lesser extent, it also acts as a neurotransmitter in the human central nervous system. In mammals, phenethylamine is produced from the amino acid L-phenylalanine by the enzyme aromatic L-amino acid decarboxylase via enzymatic decarboxylation. In addition to its presence in mammals, phenethylamine is found in many other organisms and foods, such as chocolate, especially after microbial fermentation.

Phenylpropanolamine (PPA), sold under many brand names, is a sympathomimetic agent which is used as a decongestant and appetite suppressant. It was previously commonly used in prescription and over-the-counter cough and cold preparations. The medication is taken by mouth.

Selegiline, also known as L-deprenyl and sold under the brand names Eldepryl, Zelapar, and Emsam among others, is a medication which is used in the treatment of Parkinson's disease and major depressive disorder. It has also been studied and used off-label for a variety of other indications, but has not been formally approved for any other use. The medication, in the form licensed for depression, has modest effectiveness for this condition that is similar to that of other antidepressants. Selegiline is provided as a swallowed tablet or capsule or an orally disintegrating tablet (ODT) for Parkinson's disease and as a patch applied to skin for depression.
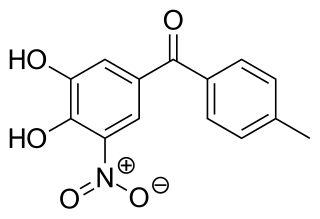
Tolcapone, sold under the brand name Tasmar, is a medication used to treat Parkinson's disease (PD). It is a selective, potent and reversible nitrocatechol-type inhibitor of the enzyme catechol-O-methyltransferase (COMT). It has demonstrated significant liver toxicity, which has led to suspension of marketing authorisations in a number of countries.
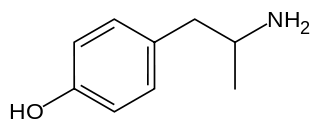
Hydroxyamphetamine, also known as 4-hydroxyamphetamine or norpholedrine and sold under the brand names Paredrine and Paremyd among others, is a sympathomimetic medication used in eye drops to dilate the pupil for eye examinations.

Rasagiline, sold under the brand name Azilect among others, is a medication which is used in the treatment of Parkinson's disease. It is used as a monotherapy to treat symptoms in early Parkinson's disease or as an adjunct therapy in more advanced cases. The drug is taken by mouth.

Pargyline, sold under the brand name Eutonyl among others, is a monoamine oxidase inhibitor (MAOI) medication which has been used to treat hypertension but is no longer marketed. It has also been studied as an antidepressant, but was never licensed for use in the treatment of depression. The drug is taken by mouth.

Molindone, sold under the brand name Moban, is an antipsychotic medication which is used in the United States in the treatment of schizophrenia. It is taken by mouth.

Lisdexamfetamine, sold under the brand names Vyvanse and Elvanse among others, is a stimulant medication that is used to treat attention deficit hyperactivity disorder (ADHD) in children and adults and for moderate-to-severe binge eating disorder in adults. Lisdexamfetamine is taken by mouth. Its effects generally begin within two hours and last for up to 14 hours.

Levodopa, also known as L-DOPA and sold under many brand names, is a dopaminergic medication which is used in the treatment of Parkinson's disease and certain other conditions like dopamine-responsive dystonia and restless legs syndrome. The drug is usually used and formulated in combination with a peripherally selective aromatic L-amino acid decarboxylase (AAAD) inhibitor like carbidopa or benserazide. Levodopa is taken by mouth, by inhalation, through an intestinal tube, or by administration into fat.

Levoamphetamine is a stimulant medication which is used in the treatment of certain medical conditions. It was previously marketed by itself under the brand name Cydril, but is now available only in combination with dextroamphetamine in varying ratios under brand names like Adderall and Evekeo. The drug is known to increase wakefulness and concentration in association with decreased appetite and fatigue. Pharmaceuticals that contain levoamphetamine are currently indicated and prescribed for the treatment of attention deficit hyperactivity disorder (ADHD), obesity, and narcolepsy in some countries. Levoamphetamine is taken by mouth.
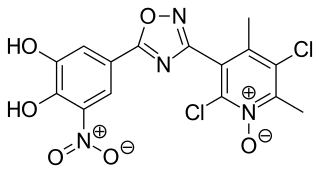
Opicapone, sold under the brand name Ongentys, is a medication which is administered together with levodopa in people with Parkinson's disease. Opicapone is a catechol-O-methyltransferase (COMT) inhibitor.

Desmethylselegiline (DMS), also known as norselegiline or as N-propargyl-L-amphetamine, is an active metabolite of selegiline, a medication used in the treatment of Parkinson's disease and depression.

The pharmacology of selegiline pertains to the pharmacodynamic and pharmacokinetic properties of the antiparkinsonian and antidepressant selegiline (L-deprenyl). Selegiline is available in a few different forms, including oral tablets and capsules, orally disintegrating tablets (ODTs), and transdermal patches. These forms have differing pharmacological properties.

A neurotransmitter prodrug, or neurotransmitter precursor, is a drug that acts as a prodrug of a neurotransmitter. A variety of neurotransmitter prodrugs have been developed and used in medicine. They can be useful when the neurotransmitter itself is not suitable for use as a pharmaceutical drug owing to unfavorable pharmacokinetic or physicochemical properties, for instance high susceptibility to metabolism, short elimination half-life, or lack of blood–brain barrier permeability. Besides their use in medicine, neurotransmitter prodrugs have also been used as recreational drugs in some cases.

O,O′-Diacetyldopamine, or 3,4-O-diacetyldopamine, also known as 3,4-diacetoxyphenethylamine, is a synthetic derivative of dopamine in which both of the hydroxyl groups have been acetylated.

O,O′-Dipivaloyldopamine, or simply dipivaloyldopamine, also known as 3,4-dipivaloyloxyphenethylamine, is a synthetic derivative of dopamine in which both of the hydroxyl groups have been acetylated. It was developed as a lipophilic prodrug of dopamine that would allow for entry of dopamine into the central nervous system.



















