In organic chemistry, acetyl is a functional group with the chemical formula −COCH3 and the structure −C(=O)−CH3. It is sometimes represented by the symbol Ac. In IUPAC nomenclature, acetyl is called ethanoyl.

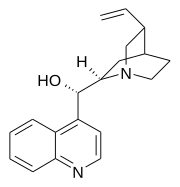
Quinoline is a heterocyclic aromatic organic compound with the chemical formula C9H7N. It is a colorless hygroscopic liquid with a strong odor. Aged samples, especially if exposed to light, become yellow and later brown. Quinoline is only slightly soluble in cold water but dissolves readily in hot water and most organic solvents. Quinoline itself has few applications, but many of its derivatives are useful in diverse applications. A prominent example is quinine, an alkaloid found in plants. Over 200 biologically active quinoline and quinazoline alkaloids are identified. 4-Hydroxy-2-alkylquinolines (HAQs) are involved in antibiotic resistance.
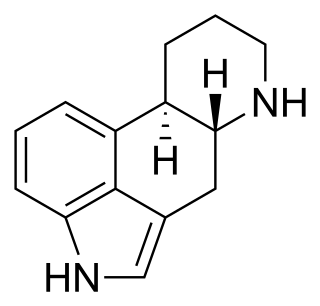
Ergoline is a chemical compound whose structural skeleton is contained in a variety of alkaloids, referred to as ergoline derivatives or ergoline alkaloids. Ergoline alkaloids, one being ergine, were initially characterized in ergot. Some of these are implicated in the condition ergotism, which can take a convulsive form or a gangrenous form. Even so, many ergoline alkaloids have been found to be clinically useful. Annual world production of ergot alkaloids has been estimated at 5,000–8,000 kg of all ergopeptines and 10,000–15,000 kg of lysergic acid, used primarily in the manufacture of semi-synthetic derivatives.
Isoxazole is an electron-rich azole with an oxygen atom next to the nitrogen. It is also the class of compounds containing this ring. Isoxazolyl is the univalent radical derived from isoxazole.
Quinazoline is an organic compound with the formula C8H6N2. It is an aromatic heterocycle with a bicyclic structure consisting of two fused six-membered aromatic rings, a benzene ring and a pyrimidine ring. It is a light yellow crystalline solid that is soluble in water. Also known as 1,3-diazanaphthalene, quinazoline received its name from being an aza derivative of quinoline. Though the parent quinazoline molecule is rarely mentioned by itself in technical literature, substituted derivatives have been synthesized for medicinal purposes such as antimalarial and anticancer agents. Quinazoline is a planar molecule. It is isomeric with the other diazanaphthalenes of the benzodiazine subgroup: cinnoline, quinoxaline, and phthalazine. Over 200 biologically active quinazoline and quinoline alkaloids are identified.
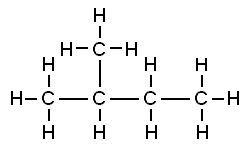
Isopentane, also called methylbutane or 2-methylbutane, is a branched-chain saturated hydrocarbon with five carbon atoms, with formula C
5H
12 or CH(CH
3)
2(C
2H
5).

Morphinan is the prototype chemical structure of a large chemical class of psychoactive drugs, consisting of opiate analgesics, cough suppressants, and dissociative hallucinogens, among others. Typical examples include compounds such as morphine, codeine, and dextromethorphan (DXM). Despite related molecular structures, the pharmacological profiles and mechanisms of action between the various types of morphinan substances can vary substantially. They tend to function either as μ-opioid receptor agonists (opioids), or NMDA receptor antagonists (dissociatives).

The δ-opioid receptor, also known as delta opioid receptor or simply delta receptor, abbreviated DOR or DOP, is an inhibitory 7-transmembrane G-protein coupled receptor coupled to the G protein Gi/G0 and has enkephalins as its endogenous ligands. The regions of the brain where the δ-opioid receptor is largely expressed vary from species model to species model. In humans, the δ-opioid receptor is most heavily expressed in the basal ganglia and neocortical regions of the brain.

Honokiol is a lignan isolated from the bark, seed cones, and leaves of trees belonging to the genus Magnolia. It has been identified as one of the chemical compounds in some traditional eastern herbal medicines along with magnolol, 4-O-methylhonokiol, and obovatol.
Imidazoline receptors are the primary receptors on which clonidine and other imidazolines act. There are three main classes of imidazoline receptor: I1 is involved in inhibition of the sympathetic nervous system to lower blood pressure, I2 has as yet uncertain functions but is implicated in several psychiatric conditions, and I3 regulates insulin secretion.
In chemical nomenclature, a preferred IUPAC name (PIN) is a unique name, assigned to a chemical substance and preferred among the possible names generated by IUPAC nomenclature. The "preferred IUPAC nomenclature" provides a set of rules for choosing between multiple possibilities in situations where it is important to decide on a unique name. It is intended for use in legal and regulatory situations.

Tebanicline is a potent synthetic nicotinic (non-opioid) analgesic drug developed by Abbott. It was developed as a less toxic analog of the potent poison dart frog-derived compound epibatidine, which is about 200 times stronger than morphine as an analgesic, but produces extremely dangerous toxic side effects. Like epibatidine, tebanicline showed potent analgesic activity against neuropathic pain in both animal and human trials, but with far less toxicity than its parent compound. It acts as a partial agonist at neuronal nicotinic acetylcholine receptors, binding to both the α3β4 and the α4β2 subtypes.

Epiboxidine is a chemical compound which acts as a partial agonist at neural nicotinic acetylcholine receptors, binding to both the α3β4 and the α4β2 subtypes. It was developed as a less toxic analogue of the potent frog-derived alkaloid epibatidine, which is around 200 times stronger than morphine as an analgesic but produces extremely dangerous toxic nicotinic side effects.

4-NEMD is a potent sedative drug which acts as a selective alpha-2 adrenergic agonist. It is closely related to dexmedetomidine but is several times more potent. Like other alpha-2 agonists, it produces sedative and muscle relaxant effects but without producing respiratory depression. It is not currently used in medicine but has been researched as the basis for a potential new generation of alpha-2 agonist drugs, which may have selectivity for the different subtypes of the alpha-2 receptor. It has two isomers, with the (S) isomer being the more potent, as with medetomidine. 4-NEMD was also investigated by the United States military as an anaesthetic agent, most likely for use in surgery but possibly also for use as a non-lethal incapacitating agent, although this has not been officially confirmed.
In pharmacology and biochemistry, allosteric modulators are a group of substances that bind to a receptor to change that receptor's response to stimuli. Some of them, like benzodiazepines or alcoholic beverages, function as psychoactive drugs. The site that an allosteric modulator binds to is not the same one to which an endogenous agonist of the receptor would bind. Modulators and agonists can both be called receptor ligands.

A-84543 is a drug developed by Abbott, which acts as an agonist at neural nicotinic acetylcholine receptors with high selectivity for the α4β2 subtype. It is widely used in scientific research into the structure and function of this receptor subtype and has been the lead compound for the development of a large family of related derivatives.
In chemistry, binding selectivity is defined with respect to the binding of ligands to a substrate forming a complex. Binding selectivity describes how a ligand may bind more preferentially to one receptor than another. A selectivity coefficient is the equilibrium constant for the reaction of displacement by one ligand of another ligand in a complex with the substrate. Binding selectivity is of major importance in biochemistry and in chemical separation processes.
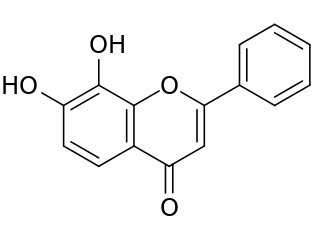
Tropoflavin, also known as 7,8-dihydroxyflavone, is a naturally occurring flavone found in Godmania aesculifolia, Tridax procumbens, and primula tree leaves. It has been found to act as a potent and selective small-molecule agonist of the tropomyosin receptor kinase B (TrkB), the main signaling receptor of the neurotrophin brain-derived neurotrophic factor (BDNF). Tropoflavin is both orally bioavailable and able to penetrate the blood–brain barrier. A prodrug of tropoflavin with greatly improved potency and pharmacokinetics, R13, is under development for the treatment of Alzheimer's disease.

ZLY18 is an experimental drug that acts as an agonist of the free fatty acid receptor 1 (FFA1) and all three types of peroxisome proliferator-activated receptor. It is in development for the treatment of non-alcoholic fatty liver disease.