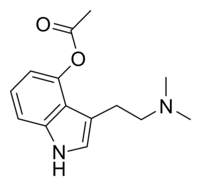
N,N-Dimethyltryptamine is a substituted tryptamine that occurs in many plants and animals, including humans, and which is both a derivative and a structural analog of tryptamine. DMT is used as a psychedelic drug and prepared by various cultures for ritual purposes as an entheogen.

Psilocybin, also known as 4-phosphoryloxy-N,N-dimethyltryptamine (4-PO-DMT), and formerly sold under the brand name Indocybin, is a naturally occurring psychedelic prodrug compound produced by more than 200 species of fungi. Psilocybin is itself biologically inactive but is quickly converted by the body to psilocin, which has mind-altering effects similar, in some aspects, to those of other classical psychedelics. Effects include euphoria, hallucinations, changes in perception, a distorted sense of time, and perceived spiritual experiences. It can also cause adverse reactions such as nausea and panic attacks.

Psilocybin mushrooms, commonly known as magic mushrooms,shrooms, or broadly as hallucinogenic mushrooms, are a polyphyletic informal group of fungi that contain psilocybin, which turns into psilocin upon ingestion. The most potent species are members of genus Psilocybe, such as P. azurescens, P. semilanceata, and P. cyanescens, but psilocybin has also been isolated from approximately a dozen other genera, including Panaeolus, Inocybe, Pluteus, Gymnopilus, and Pholiotina.
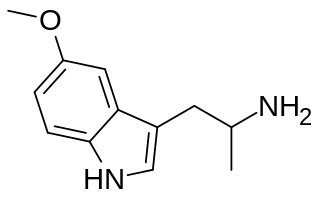
5-MeO-αMT, or 5-methoxy-α-methyltryptamine, also known as α,O-dimethylserotonin (Alpha-O), is a serotonergic psychedelic of the tryptamine family. It is a derivative of α-methyltryptamine (αMT) and an analogue of 5-MeO-DMT.
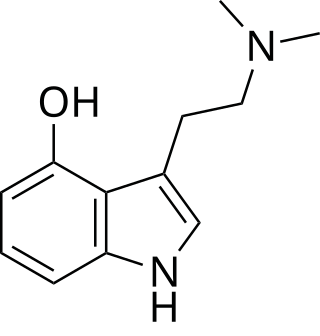
Psilocin, also known as 4-hydroxy-N,N-dimethyltryptamine (4-OH-DMT), is a substituted tryptamine alkaloid and a serotonergic psychedelic. It is present in most psychedelic mushrooms together with its phosphorylated counterpart psilocybin.

Bufotenin, also known as dimethylserotonin or as 5-hydroxy-N,N-dimethyltryptamine (5-HO-DMT), is a serotonergic psychedelic of the tryptamine family. It is a derivative of the psychedelic dimethyltryptamine (DMT) and of the neurotransmitter serotonin. The compound is an alkaloid found in some species of mushrooms, plants, and toads. It is also found naturally in the human body in small amounts. Bufotenin, for instance derived from the trees Anadenanthera colubrina and Anadenanthera peregrina, appears to have a long history of entheogenic use in South America.

DET, also known under its chemical name N,N-diethyltryptamine and as T-9, is a psychedelic drug closely related to DMT and 4-HO-DET. However, despite its structural similarity to DMT, its activity is induced by an oral dose of around 50–100 mg, without the aid of MAO inhibitors, and the effects last for about 2–4 hours.
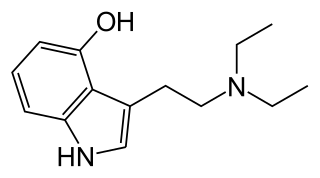
4-HO-DET, also known as 4-hydroxy-diethyl-tryptamine, CZ-74, is a hallucinogenic drug and psychedelic compound of moderate duration. 4-HO-DET is a substituted tryptamine, structurally related to psilocin, ethocybin, and 4-HO-DIPT.

Baeocystin, also known as norpsilocybin or 4-phosphoryloxy-N-methyltryptamine (4-PO-NMT), is a zwitterionic alkaloid and analogue of psilocybin. It is found as a minor compound in most psilocybin mushrooms together with psilocybin, norbaeocystin, aeruginascin, and psilocin. Baeocystin is the N-demethylated derivative of psilocybin and the 4-phosphorylated derivative of 4-HO-NMT (4-hydroxy-N-methyltryptamine). The structures at right illustrate baeocystin in its zwitterionic form.

4-HO-MiPT is a synthetic substituted aromatic compound and a lesser-known psychedelic tryptamine. It is thought to be a serotonergic psychedelic, similar to magic mushrooms, LSD and mescaline. Its molecular structure and pharmacological effects somewhat resemble those of the tryptamine psilocin, which is the primary psychoactive chemical in magic mushrooms.

Ethocybin is a homologue of the mushroom alkaloid psilocybin, and a semi-synthetic psychedelic alkaloid of the tryptamine family. Effects of ethocybin are comparable to those of a shorter LSD or psilocybin trip, although intensity and duration vary depending on dosage, individual physiology, and set and setting.
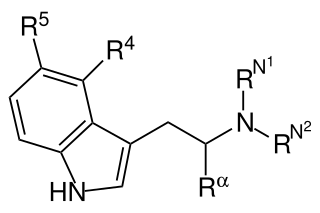
Substituted tryptamines, or simply tryptamines, also known as serotonin analogues (i.e., 5-hydroxytryptamine analogues), are organic compounds which may be thought of as being derived from tryptamine itself. The molecular structures of all tryptamines contain an indole ring, joined to an amino (NH2) group via an ethyl (−CH2–CH2−) sidechain. In substituted tryptamines, the indole ring, sidechain, and/or amino group are modified by substituting another group for one of the hydrogen (H) atoms.

4-acetoxy-MET (4-acetoxy-N-methyl-N-ethyltryptamine), also known as 4-AcO-MET or metacetin, is a hallucinogenic tryptamine. It is the acetate ester of 4-HO-MET, and a homologue of 4-AcO-DMT. It is a novel compound with very little history of human use. It is sometimes sold as a research chemical by online retailers.
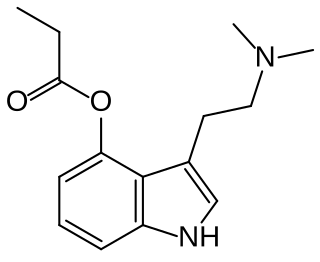
4-Propionoxy-N,N-dimethyltryptamine is a synthetic psychedelic drug from the tryptamine family with psychedelic effects, and is believed to act as a prodrug for psilocin. It produces a head-twitch response in mice. It has been sold online as a designer drug since May 2019. It was first identified as a new psychoactive substance in Sweden, in July 2019. A number of related derivatives have been synthesized as prodrugs of psilocin for medical applications.
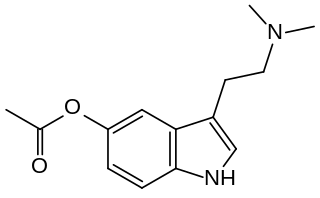
O-Acetylbufotenine, or bufotenine O-acetate, also known as 5-acetoxy-N,N-dimethyltryptamine (5-AcO-DMT) or O-acetyl-N,N-dimethylserotonin, is a synthetic tryptamine derivative and putative serotonergic psychedelic. It is the O-acetylated analogue of the naturally occurring peripherally selective serotonergic tryptamine bufotenine and is thought to act as a centrally penetrant prodrug of bufotenine.

4-HO-TMT, or 4-OH-TMT, also known as 4-hydroxy-N,N,N-trimethyltryptammonium or as dephosphorylated aeruginascin, is a substituted tryptamine derivative and the active form of aeruginascin (4-PO-TMT), analogously to how psilocin (4-HO-DMT) is the active form of psilocybin (4-PO-DMT). 4-HO-TMT is closely related to bufotenidine, the N-trimethyl analogue of serotonin.

O-Pivalylbufotenine, or bufotenine O-pivalate, also known as 5-pivaloxy-N,N-dimethyltryptamine or O-pivalyl-N,N-dimethylserotonin, is a synthetic tryptamine derivative and putative serotonergic psychedelic. It is the O-pivalyl analogue of the naturally occurring but peripherally selective serotonergic tryptamine bufotenine and is thought to act as a centrally penetrant prodrug of bufotenine.

4-Hydroxytryptamine, also known as N,N-didesmethylpsilocin, is a naturally occurring tryptamine alkaloid. It is closely related chemically to the neurotransmitter serotonin, the psychedelic psilocin, and is the active form of the tryptamine alkaloid norbaeocystin.
Diamond Shruumz, also sometimes referred to as Diamond Shrooms, is a brand of mushroom edibles sold by Prophet Premium Blends, a company located in Santa Ana, California in the United States. It includes chocolate bar, gummy, and candy cone products. The products are marketed with words such as "magic", "nootropic", and "microdosing". They do not include a full list of ingredients, but are listed as containing a proprietary "mushroom blend". Diamond Shruumz products are sold both online and in some retail stores such as vape shops in the United States.
A mushroom edible, also sometimes known as "legal shrooms", is a food item that may contain hallucinogens associated with those in psychoactive mushrooms, such as psilocybin mushrooms or Amanita muscaria mushrooms. They include chocolate bars and gummies, among others.