
Nalbuphine, sold under the brand names Nubain among others, is an opioid analgesic which is used in the treatment of pain. It is given by injection into a vein, muscle, or fat.

An opioid antagonist, or opioid receptor antagonist, is a receptor antagonist that acts on one or more of the opioid receptors.

Butorphanol is a morphinan-type synthetic agonist–antagonist opioid analgesic developed by Bristol-Myers. Butorphanol is most closely structurally related to levorphanol. Butorphanol is available as the tartrate salt in injectable, tablet, and intranasal spray formulations. The tablet form is only used in dogs, cats and horses due to low bioavailability in humans.

Levorphanol is an opioid medication used to treat moderate to severe pain. It is the levorotatory enantiomer of the compound racemorphan. Its dextrorotatory counterpart is dextrorphan.

The κ-opioid receptor or kappa opioid receptor, abbreviated KOR or KOP for its ligand ketazocine, is a G protein-coupled receptor that in humans is encoded by the OPRK1 gene. The KOR is coupled to the G protein Gi/G0 and is one of four related receptors that bind opioid-like compounds in the brain and are responsible for mediating the effects of these compounds. These effects include altering nociception, consciousness, motor control, and mood. Dysregulation of this receptor system has been implicated in alcohol and drug addiction.
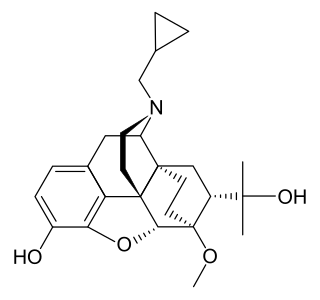
Diprenorphine, also known as diprenorfin, is a non-selective, high-affinity, weak partial agonist of the μ- (MOR), κ- (KOR), and δ-opioid receptor (DOR) which is used in veterinary medicine as an opioid antagonist. It is used to reverse the effects of super-potent opioid analgesics such as etorphine and carfentanil that are used for tranquilizing large animals. The drug is not approved for use in humans.

7-Hydroxymitragynine is a terpenoid indole alkaloid from the plant Mitragyna speciosa, commonly known as kratom. It is often referred to as '7-OH'. It was first described in 1994 and is a natural product derived from the mitragynine present in the kratom leaf. It is considered an oxidized derivative and active metabolite of mitragynine. 7-OH binds to opioid receptors like mitragynine, but research suggests that 7-OH binds with greater potency and contributes heavily to the analgesic activity of mitragynine as a metabolite.
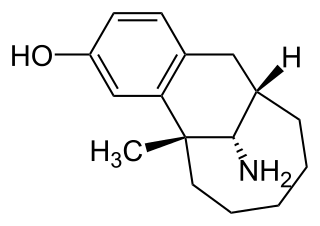
Dezocine, sold under the brand name Dalgan, is an atypical opioid analgesic which is used in the treatment of pain. It is used by intravenous infusion and intramuscular injection.
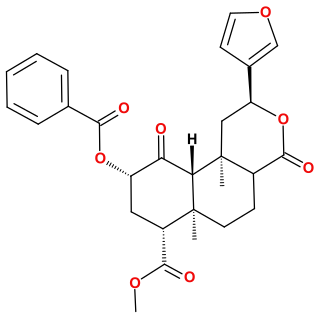
Herkinorin is an opioid analgesic that is an analogue of the natural product salvinorin A. It was discovered in 2005 during structure-activity relationship studies into neoclerodane diterpenes, the family of chemical compounds of which salvinorin A is a member.

Propiram is a partial μ-opioid receptor agonist and weak μ antagonist analgesic from the ampromide family of drugs related to other drugs such as phenampromide and diampromide. It was invented in 1963 in the United Kingdom by Bayer but was not widely marketed, although it saw some limited clinical use, especially in dentistry. Propiram reached Phase III clinical trials in the United States and Canada.

Oxilorphan is an opioid antagonist of the morphinan family that was never marketed. It acts as a μ-opioid receptor (MOR) antagonist but a κ-opioid receptor (KOR) partial agonist, and has similar effects to naloxone and around the same potency as an MOR antagonist. Oxilorphan has some weak partial agonist actions at the MOR and can produce hallucinogenic/dissociative effects at sufficient doses, indicative of KOR activation. It was trialed for the treatment of opioid addiction, but was not developed commercially. The KOR agonist effects of oxilorphan are associated with dysphoria, which combined with its hallucinogenic effects, serve to limit its clinical usefulness; indeed, many patients who experienced these side effects refused to take additional doses in clinical trials.

Levallorphan, also known as levallorphan tartrate (USAN), is an opioid modulator of the morphinan family used as an opioid analgesic and opioid antagonist/antidote. It acts as an antagonist of the μ-opioid receptor (MOR) and as an agonist of the κ-opioid receptor (KOR), and as a result, blocks the effects of stronger agents with greater intrinsic activity such as morphine whilst simultaneously producing analgesia.

Normorphine is an opiate analogue, the N-demethylated derivative of morphine, that was first described in the 1950s when a large group of N-substituted morphine analogues were characterized for activity. The compound has relatively little opioid activity in its own right, but is a useful intermediate which can be used to produce both opioid antagonists such as nalorphine, and also potent opioid agonists such as N-phenethylnormorphine. with its formation from morphine catalyzed by the liver enzymes CYP3A4 and CYP2C8.

Xorphanol (INN), also known as xorphanol mesylate (USAN), is an opioid analgesic of the morphinan family that was never marketed.
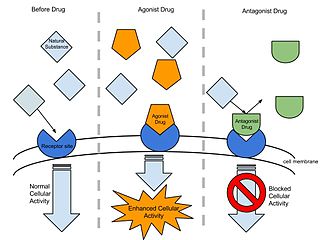
In pharmacology the term agonist-antagonist or mixed agonist/antagonist is used to refer to a drug which under some conditions behaves as an agonist while under other conditions, behaves as an antagonist.

Alazocine, also known more commonly as N-allylnormetazocine (NANM), is a synthetic opioid analgesic of the benzomorphan family related to metazocine, which was never marketed. In addition to its opioid activity, the drug is a sigma receptor agonist, and has been used widely in scientific research in studies of this receptor. Alazocine is described as a potent analgesic, psychotomimetic or hallucinogen, and opioid antagonist. Moreover, one of its enantiomers was the first compound that was found to selectively label the σ1 receptor, and led to the discovery and characterization of the receptor.

MT-45 (IC-6) is an opioid analgesic drug invented in the 1970s by Dainippon Pharmaceutical Co. It is chemically a 1-substituted-4-(1,2-diphenylethyl) piperazine derivative, which is structurally unrelated to most other opioid drugs. Racemic MT-45 has around 80% the potency of morphine, with almost all opioid activity residing in the (S) enantiomer. It has been used as a lead compound from which a large family of potent opioid drugs have been developed, including full agonists, partial agonists, and antagonists at the three main opioid receptor subtypes. Fluorinated derivatives of MT-45 such as 2F-MT-45 are significantly more potent as μ-opioid receptor agonists, and one of its main metabolites 1,2-diphenylethylpiperazine also blocks NMDA receptors.

8-Carboxamidocyclazocine (8-CAC) is an opioid analgesic drug related to cyclazocine, discovered by medicinal chemist Mark P. Wentland and co-workers in Cogswell Laboratory at Rensselaer Polytechnic Institute. Similarly to cyclazocine, 8-CAC acts as an agonist at both the μ- and κ-opioid receptors, but has a much longer duration of action than cyclazocine, and does not have μ antagonist activity. Unexpectedly, it was discovered that the phenolic hydroxyl group of cyclazocine could be replaced by a carboxamido group with only slight loss of potency at opioid receptors, and this discovery has subsequently been used to develop many novel opioid derivatives where the phenolic hydroxy group has been replaced by either carboxamide or a variety of larger groups. Due to their strong κ-opioid agonist activity, these drugs are not suited for use as analgesics in humans, but have instead been researched as potential drugs for the treatment of cocaine addiction.
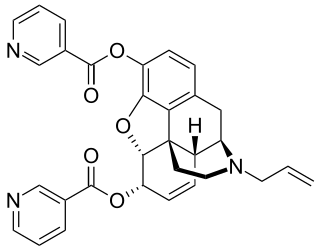
Nalorphine dinicotinate, also known as N-allylnormorphine dinicotinate, dinicotinoylnalorphine, or niconalorphine, is a semisynthetic, mixed opioid agonist-antagonist which is described as a narcotic antagonist but may produce limited analgesia and sedation at higher doses in opioid naive patients. It is the 3,6-dinicotinate ester of nalorphine, and is therefore the nalorphine analogue of nicomorphine.

Nalodeine, also known more commonly as N-allylnorcodeine, is an opioid antagonist that was never marketed but is notable as the first opioid antagonist to be discovered. It was first reported in 1915 and was found to block the effects of morphine in animals. This was followed by the clinical introduction of nalorphine (N-allylnormorphine) in 1954, naloxone (N-allyloxymorphone) in 1960, and naltrexone (N-methylcyclopropyloxymorphone) in 1963. Nalmefene (6-desoxy-6-methylene-naltrexone), another structurally related opioid antagonist derivative, was also subsequently introduced, in 1996. In animals, nalodeine both reverses morphine- and heroin-induced respiratory depression and acts as a respiratory stimulant in its own right. Similarly to nalorphine, nalodeine has also been found to act as an agonist of the κ-opioid receptor.





















