| |
| Names | |
|---|---|
| Preferred IUPAC name Ethyl carbonochloridate | |
Other names
| |
| Identifiers | |
3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.007.981 |
| EC Number |
|
PubChem CID | |
| RTECS number |
|
| UNII | |
| UN number | 1182 |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| ClCO2CH2CH3 | |
| Molar mass | 108.52 g·mol−1 |
| Appearance | Colorless liquid |
| Odor | Like hydrochloric acid [2] |
| Density | 1.1403 g/cm3 |
| Melting point | −81 °C (−114 °F; 192 K) |
| Boiling point | 95 °C (203 °F; 368 K) |
| Decomposes | |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards | Corrosive Flammable |
| GHS labelling: | |
    | |
| Danger | |
| H225, H302, H314, H330 | |
| P210, P233, P240, P241, P242, P243, P260, P264, P270, P271, P280, P284, P301+P312, P301+P330+P331, P303+P361+P353, P304+P340, P305+P351+P338, P310, P320, P321, P330, P363, P370+P378, P403+P233, P403+P235, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Flash point | 61 °C (142 °F; 334 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
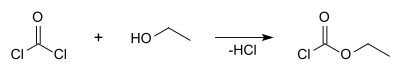
Ethyl chloroformate is an organic compound with the chemical formula ClCO2CH2CH3. It is the ethyl ester of chloroformic acid. It is a colorless, corrosive and highly toxic liquid. It is a reagent used in organic synthesis for the introduction of the ethyl carbamate protecting group [3] and for the formation of carboxylic anhydrides.