
25I-NBOH is a derivative of the phenethylamine-derived hallucinogen 2C-I that was discovered in 2006 by a team at Purdue University.

25I-NBOMe is a novel synthetic psychoactive substance with strong hallucinogenic properties, synthesized in 2003 for research purposes. Since 2010, it has circulated in the recreational drug scene, often misrepresented as LSD. The recreational usage of 25I is associated with severe intoxication and deaths in humans.
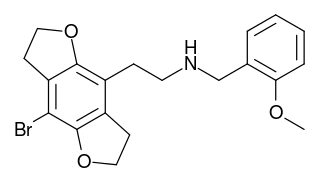
2CBFly-NBOMe is a compound indirectly derived from the phenethylamine hallucinogen 2C-B, and related to benzodifurans like 2C-B-FLY and N-benzylphenethylamines like 25I-NBOMe. It was discovered in 2002, and further researched by Ralf Heim at the Free University of Berlin, and subsequently investigated in more detail by a team at Purdue University led by David E. Nichols. It acts as a potent partial agonist for the 5HT2A serotonin receptor subtype.

25I-NBMD is a derivative of the phenethylamine hallucinogen 2C-I, discovered in 2006 by a team at Purdue University led by David Nichols. It acts as a potent partial agonist for the 5HT2A receptor with a Ki of 0.049 nM at the human 5HT2A receptor. The corresponding 4-bromo analogue 25B-NBMD has been used for molecular dynamics studies on the shape of the 5-HT2A receptor.

25B-NBOMe is a derivative of the phenethylamine psychedelic 2C-B, discovered in 2004 by Ralf Heim at the Free University of Berlin. It acts as a potent full agonist for the 5HT2A receptor. Anecdotal reports from users suggest 25B-NBOMe to be an active hallucinogen at a dose of as little as 250–500 µg, making it a similar potency to other phenethylamine derived hallucinogens such as Bromo-DragonFLY. Duration of effects lasts about 12–16 hours, although the parent compound is rapidly cleared from the blood when used in the radiolabeled form in tracer doses. Recently, Custodio et al (2019) evaluated the potential involvement of dysregulated dopaminergic system, neuroadaptation, and brain wave changes which may contribute to the rewarding and reinforcing properties of 25B-NBOMe in rodents.

25TFM-NBOMe is a derivative of the phenethylamine hallucinogen 2C-TFM, discovered in 2004 by Ralf Heim at the Free University of Berlin. It acts as a potent partial agonist for the 5HT2A receptor, though its relative potency is disputed, with some studies finding it to be of lower potency than 25I-NBOMe, while others show it to be of similar or higher potency, possibly because of differences in the assay used. 2C-TFM-NB2OMe can be taken to produce psychedelic effects similar to 2C-I-NB2OMe and 2C-D-NB2OMe.
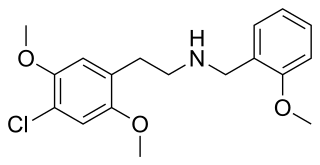
25C-NBOMe is a psychedelic drug and derivative of the psychedelic phenethylamine 2C-C. 25C-NBOMe appeared on online vendor sites in 2010 but was not reported in the literature until 2011. It acts as a potent agonist of the 5HT2A receptor, and has been studied in its 11C radiolabelled form as a potential ligand for mapping the distribution of 5-HT2A receptors in the brain, using positron emission tomography (PET). Multiple deaths have occurred from usage of 25C-NBOMe due to the ease of accidental overdose. The long-term toxic effects of the drug have not been researched.

25D-NBOMe is a derivative of the phenethylamine derived hallucinogen 2C-D. It acts in a similar manner to related compounds such as 25I-NBOMe, which is a potent agonist at the 5HT2A receptor. 25D-NBOMe has been sold as a street drug since 2010 and produces similar effects in humans to related compounds such as 25I-NBOMe and 25C-NBOMe. It was banned as a Temporary Class Drug in the UK on 10 June 2013 after concerns about its recreational use.

25-C-NBOH is a derivative of the phenethylamine derived hallucinogen 2C-C which has been sold as a designer drug. It has similar serotonin receptor affinity to the better-known compound 25C-NBOMe.

25CN-NBOH is a compound indirectly derived from the phenethylamine series of hallucinogens, which was discovered in 2014 at the University of Copenhagen. This compound is notable as one of the most selective agonist ligands for the 5-HT2A receptor yet discovered, with a pKi of 8.88 at the human 5-HT2A receptor and with 100x selectivity for 5-HT2A over 5-HT2C, and 46x selectivity for 5-HT2A over 5-HT2B. A tritiated version of 25CN-NBOH has also been accessed and used for more detailed investigations of the binding to 5-HT2 receptors and autoradiography.

25CN-NBOMe is a derivative of the phenethylamine 2C-CN. It acts in a similar manner to related compounds such as 25I-NBOMe, which are potent agonists at the 5HT2A receptor.

25N-NBOMe is a derivative of the hallucinogen 2C-N. The pharmacological properties of 25N-NBOMe have not been described in the scientific literature, but it is believed to act in a similar manner to related compounds such as 25I-NBOMe and 25C-NBOMe, which are potent agonists at the 5HT2A receptor. 25N-NBOMe has been sold as a street drug and has only been described in the literature in terms of identification by forensic analysis.

25E-NBOMe is a derivative of the phenethylamine 2C-E. It acts in a similar manner to related compounds such as 25I-NBOMe, which are potent agonists at the 5HT2A receptor. 25E-NBOMe has been sold as a drug and produces similar effects in humans to related compounds such as 25I-NBOMe and 25C-NBOMe.
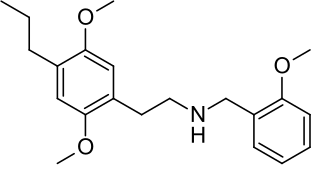
25P-NBOMe is a derivative of the phenethylamine 2C-P. It acts in a similar manner to related compounds such as 25I-NBOMe, which are potent agonists at the 5HT2A receptor. 25P-NBOMe has been sold as a drug and produces similar effects in humans to related compounds such as 25I-NBOMe and 25C-NBOMe.

25I-NB34MD (NB34MD-2C-I) is a derivative of the phenethylamine hallucinogen 2C-I, which acts as a potent partial agonist for the human 5-HT2A receptor, and presumably has similar properties to 2C-I. It has a binding affinity of 0.67nM at the human 5-HT2A receptor, making it several times weaker than its positional isomer 25I-NBMD and a similar potency to 25I-NBF.

25H-NBOMe (NBOMe-2C-H) is a derivative of the phenethylamine hallucinogen 2C-H, which acts as a highly potent full agonist for the human 5-HT2A receptor.

25iP-NBOMe is a derivative of the phenethylamine hallucinogen 2C-iP, which acts as a highly potent agonist for the human 5-HT2A receptor.

The 25-NB (25x-NBx) series, sometimes alternatively referred to as the NBOMe compounds, is a family of serotonergic psychedelics. They are substituted phenethylamines and were derived from the 2C family. They act as selective agonists of the serotonin 5-HT2A receptor. The 25-NB family is unique relative to other classes of psychedelics in that they are, generally speaking, extremely potent and relatively selective for the 5-HT2A receptor. Use of NBOMe series drugs has caused many deaths and hospitalisations since the drugs popularisation in the 2010s. This is primarily due to their high potency, unpredictable pharmacokinetics, and sellers passing off the compounds in the series as LSD.

25E-NBOH is a derivative of the phenethylamine derived hallucinogen 2C-E. It was first developed by Martin Hansen at the University of Copenhagen in 2010 as a brain imaging agent, but has subsequently been sold as a designer drug, first being identified in Brazil in 2018 on seized blotter paper, as well as in Slovenia. It acts as a potent serotonin receptor agonist with similar affinity to better-known compounds such as 25I-NBOMe at 5-HT2A and 5-HT2C receptors.

N-Benzyl-2C-B is a recreational designer drug from the 25-NB subgroup of the substituted phenethylamine family, with psychedelic effects. It has a binding affinity (Ki) of 16 nM at the serotonin receptor 5-HT2A and 90 nM at 5-HT2C and reportedly has a potency in between that of 2C-B and NBOMe-2C-B.




















