
The 5-HT2A receptor is a subtype of the 5-HT2 receptor that belongs to the serotonin receptor family and is a G protein-coupled receptor (GPCR). The 5-HT2A receptor is a cell surface receptor, but has several intracellular locations. 5-HT is short for 5-hydroxy-tryptamine or serotonin. This is the main excitatory receptor subtype among the GPCRs for serotonin, although 5-HT2A may also have an inhibitory effect on certain areas such as the visual cortex and the orbitofrontal cortex. This receptor was first noted for its importance as a target of serotonergic psychedelic drugs such as LSD and psilocybin mushrooms. Later it came back to prominence because it was also found to be mediating, at least partly, the action of many antipsychotic drugs, especially the atypical ones.

The 5HT6 receptor is a subtype of 5HT receptor that binds the endogenous neurotransmitter serotonin (5-hydroxytryptamine, 5HT). It is a G protein-coupled receptor (GPCR) that is coupled to Gs and mediates excitatory neurotransmission. HTR6 denotes the human gene encoding for the receptor.

The 5-HT7 receptor is a member of the GPCR superfamily of cell surface receptors and is activated by the neurotransmitter serotonin (5-hydroxytryptamine, 5-HT) The 5-HT7 receptor is coupled to Gs (stimulates the production of the intracellular signaling molecule cAMP) and is expressed in a variety of human tissues, particularly in the brain, the gastrointestinal tract, and in various blood vessels. This receptor has been a drug development target for the treatment of several clinical disorders. The 5-HT7 receptor is encoded by the HTR7 gene, which in humans is transcribed into 3 different splice variants.

AL-34662 is an indazole derivative drug that is being developed for the treatment of glaucoma. It acts as a selective 5-HT2A receptor agonist, the same target as that of psychedelic drugs like psilocin, but unlike these drugs, AL-34662 was designed specifically as a peripherally selective drug, which does not cross the blood–brain barrier. This means that AL-34662 can exploit a useful side effect of the hallucinogenic 5-HT2A agonists, namely reduction in intra-ocular pressure and hence relief from the symptoms of glaucoma, but without causing the hallucinogenic effects that make centrally active 5-HT2A agonists unsuitable for clinical use. In animal studies, AL-34662 has been shown to be potent and effective in the treatment of symptoms of glaucoma, with minimal side effects.
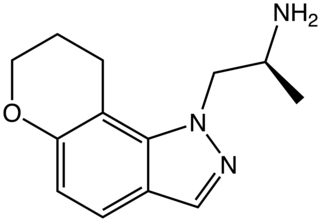
AL-38022A is an indazole derivative drug which is one of a range of similar drugs developed for scientific research and with some possible clinical applications. It acts as a potent and selective agonist for the 5-HT2 family of serotonin receptors, with highest binding affinity for the 5-HT2C subtype and around 4x less affinity for 5-HT2A and 5-HT2B. In drug discrimination tests on animals, it fully substituted for both DOM and 5-MeO-DMT.

AL-37350A (4,5-DHP-AMT) is a tryptamine derivative which acts as a potent and selective agonist for the serotonin receptor 5-HT2A, with a Ki of 2.0 nM, and moderate selectivity over the related 5-HT2B and 5-HT2C receptors. It has been shown to have ocular hypotensive activity in animal models, suggesting it may be useful for the treatment of glaucoma.

Dimemebfe (5-MeO-BFE) is a recreational drug and research chemical. It acts as an agonist for the 5-HT1A and 5-HT2 family of serotonin receptors. It is related in structure to the psychedelic tryptamine derivative 5-MeO-DMT, but with the indole nitrogen replaced by oxygen, making dimemebfe a benzofuran derivative. It is several times less potent as a serotonin agonist than 5-MeO-DMT and with relatively more activity at 5-HT1A, but still shows strongest effects at the 5-HT2 family of receptors.

5-Methoxy-7,N,N-trimethyltryptamine (5-MeO-7,N,N-TMT, 5-MeO-7-TMT), is a tryptamine derivative which acts as a partial agonist at the 5-HT2 serotonin receptors, with an EC50 of 63.9 nM and an efficacy of 66.2% at 5-HT2A (vs 5-HT), and weaker activity at 5-HT2B and 5-HT2C. In animal tests, both 7,N,N-TMT and 5-MeO-7,N,N-TMT produced behavioural responses similar to those of psychedelic drugs such as DMT and 5-MeO-DMT, but compounds with larger 7-position substituents such as 7-ethyl-DMT and 7-bromo-DMT did not produce psychedelic-appropriate responding despite high 5-HT2 receptor binding affinity, suggesting these may be antagonists or weak partial agonists for the 5-HT2 receptors. The related compound 7-MeO-MiPT (cf. 5-MeO-MiPT) was also found to be inactive, suggesting that the 7-position has poor tolerance for bulky groups at this position, at least if agonist activity is desired.

7,N,N-trimethyltryptamine (7-methyl-DMT, 7-TMT), is a tryptamine derivative which acts as an agonist of 5-HT2 receptors. In animal tests, both 7-TMT and its 5-methoxy derivative 5-MeO-7-TMT produced behavioural responses similar to those of psychedelic drugs such as DMT, but the larger 7-ethyl and 7-bromo derivatives of DMT did not produce psychedelic responses despite having higher 5-HT2 receptor affinity in vitro (cf. DOBU, DOAM). 7-TMT also weakly inhibits reuptake of serotonin but with little effect on dopamine or noradrenaline reuptake.
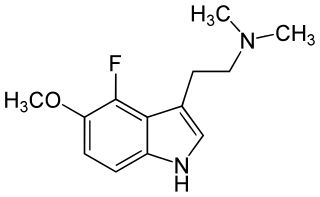
4-Fluoro-5-Methoxy-N,N-dimethyltryptamine (4-F-5-MeO-DMT) was first described by David E. Nichols team in 2000. It is a potent 5-HT1A agonist. Substitution with the 4-fluorine markedly increased 5-HT1A selectivity over 5-HT2A/2C receptors with potency greater than that of the 5-HT1A agonist 8-OH-DPAT.

CP-93129 is a drug which acts as a potent and selective serotonin 5-HT1B receptor agonist, with approximately 150x and 200x selectivity over the closely related 5-HT1D and 5-HT1A receptors. It is used in the study of 5-HT1B receptors in the brain, particularly their role in modulating the release of other neurotransmitters.
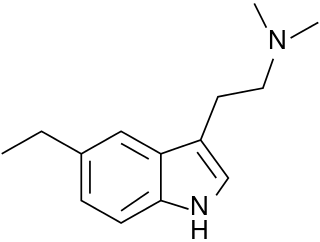
5-Ethyl-N,N-dimethyltryptamine is a tryptamine derivative which acts as an agonist at the 5-HT1A and 5-HT1D serotonin receptors, with around 3x selectivity for 5-HT1D.
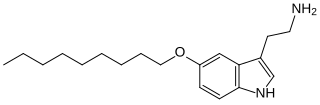
5-(Nonyloxy)tryptamine is a tryptamine derivative which acts as a selective agonist at the 5-HT1B receptor. Increasing the O-alkoxy chain length in this series gives generally increasing potency and selectivity for 5-HT1B, with highest activity found for the nonyloxy derivative, having a 5-HT1B binding affinity of 1.0 nM, and around 300-fold selectivity over the related 5-HT1A receptor.

SB-206553 is a drug which acts as a mixed antagonist for the 5-HT2B and 5-HT2C serotonin receptors. It has anxiolytic properties in animal studies and interacts with a range of other drugs. It has also been shown to act as a positive allosteric modulator of α7 nicotinic acetylcholine receptors. Modified derivatives of SB-206553 have been used to probe the structure of the 5-HT2B receptor.

Substituted tryptamines, or serotonin analogues, are organic compounds which may be thought of as being derived from tryptamine itself. The molecular structures of all tryptamines contain an indole ring, joined to an amino (NH2) group via an ethyl (−CH2–CH2−) sidechain. In substituted tryptamines, the indole ring, sidechain, and/or amino group are modified by substituting another group for one of the hydrogen (H) atoms.
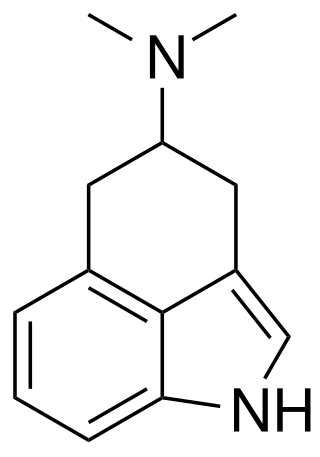
RU-28306 is a synthetic indole alkaloid derivative which acts as a serotonin receptor agonist, with selectivity for 5-HT1 and 5-HT2 subtypes. It can be regarded either as a tricyclic derivative of DMT, or a structurally simplified analogue of LSD, but the binding affinity of racemic RU-28306 is closer to that of DMT, though with relatively higher affinity for 5-HT2 subtypes and lower for 5-HT1. It has been sold as a designer drug and was first reported to the EMCDDA by a forensic laboratory in Slovenia in 2017.
5-HT2C receptor agonists are a class of drugs that activate 5-HT2C receptors. They have been investigated for the treatment of a number of conditions including obesity, psychiatric disorders, sexual dysfunction and urinary incontinence.

CP-132,484 is a tryptamine derivative which acts as a potent and selective agonist for the 5-HT2 family of serotonin receptors. It has reasonable selectivity for 5-HT2A and 5-HT2C subtypes over 5-HT2B, but is only slightly selective for 5-HT2A over 5-HT2C. This compound and several related analogues have been shown to have ocular hypotensive activity in animal models, suggesting they may be useful for the treatment of glaucoma.

5-MeO-DiBF is a psychedelic that has been sold online as a designer drug and was first definitively identified in December 2015 by a forensic laboratory in Slovenia. It is thought to act as an agonist for the 5-HT1A and 5-HT2 family of serotonin receptors. It is related in structure to the psychedelic tryptamine derivative 5-MeO-DiPT, but with the indole nitrogen replaced by oxygen, making 5-MeO-DiBF a benzofuran derivative. It is several times less potent as a serotonin agonist than 5-MeO-DiPT and with relatively more activity at 5-HT1A, but still shows strongest effects at the 5-HT2 family of receptors.

SN-22 is a chemical compound which acts as a moderately selective agonist at the 5-HT2 family of serotonin receptors, with a Ki of 19nM at 5HT2 subtypes vs 514 nM at 5-HT1A receptors. Many related derivatives are known, most of which are ligands for 5-HT1A, 5-HT6 or dopamine D2 receptors or show SSRI activity.



















