
Salvinorin A is the main active psychotropic molecule in Salvia divinorum. Salvinorin A is considered a dissociative hallucinogen.
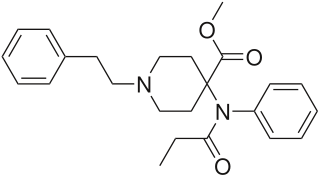
Carfentanil or carfentanyl, sold under the brand name Wildnil, is an extremely potent opioid analgesic which is used in veterinary medicine to anesthetize large animals such as elephants and rhinoceroses. It is typically administered in this context by tranquilizer dart. Carfentanil has also been used in humans for imaging of opioid receptors. It has additionally been used as a recreational drug, typically by injection, insufflation, or inhalation. Deaths have been reported in association with carfentanil.
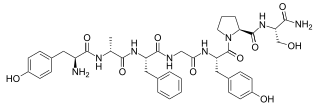
Dermorphin is a hepta-peptide first isolated from the skin of South American frogs belonging to the genus Phyllomedusa. The peptide is a natural opioid that binds as an agonist with high potency and selectivity to mu opioid receptors. Dermorphin is about 30–40 times more potent than morphine, but theoretically may be less likely to produce drug tolerance and addiction due to its high potency. The amino acid sequence of dermorphin is H-Tyr-D-Ala-Phe-Gly-Tyr-Pro-Ser-NH2.

Opioid peptides or opiate peptides are peptides that bind to opioid receptors in the brain; opiates and opioids mimic the effect of these peptides. Such peptides may be produced by the body itself, for example endorphins. The effects of these peptides vary, but they all resemble those of opiates. Brain opioid peptide systems are known to play an important role in motivation, emotion, attachment behaviour, the response to stress and pain, control of food intake, and the rewarding effects of alcohol and nicotine.

Phyllomedusa bicolor, the giant leaf frog, bicolor tree-frog, giant monkey frog, or waxy-monkey treefrog, is a species of leaf frog. It can be found in the Amazon basin of Brazil, Colombia (Amazonas), Bolivia, and Peru, and can also be found in the Guianan Region of Venezuela and the Guianas, and in Cerrado of the state of Maranhão in Brazil.

The sigma-1 receptor (σ1R), one of two sigma receptor subtypes, is a chaperone protein at the endoplasmic reticulum (ER) that modulates calcium signaling through the IP3 receptor. In humans, the σ1 receptor is encoded by the SIGMAR1 gene.

Met-enkephalin, also known as metenkefalin (INN), sometimes referred to as opioid growth factor (OGF), is a naturally occurring, endogenous opioid peptide that has opioid effects of a relatively short duration. It is one of the two forms of enkephalin, the other being leu-enkephalin. The enkephalins are considered to be the primary endogenous ligands of the δ-opioid receptor, due to their high potency and selectivity for the site over the other endogenous opioids.

The nociceptin opioid peptide receptor (NOP), also known as the nociceptin/orphanin FQ (N/OFQ) receptor or kappa-type 3 opioid receptor, is a protein that in humans is encoded by the OPRL1 gene. The nociceptin receptor is a member of the opioid subfamily of G protein-coupled receptors whose natural ligand is the 17 amino acid neuropeptide known as nociceptin (N/OFQ). This receptor is involved in the regulation of numerous brain activities, particularly instinctive and emotional behaviors. Antagonists targeting NOP are under investigation for their role as treatments for depression and Parkinson's disease, whereas NOP agonists have been shown to act as powerful, non-addictive painkillers in non-human primates.

The δ-opioid receptor, also known as delta opioid receptor or simply delta receptor, abbreviated DOR or DOP, is an inhibitory 7-transmembrane G-protein coupled receptor coupled to the G protein Gi/G0 and has enkephalins as its endogenous ligands. The regions of the brain where the δ-opioid receptor is largely expressed vary from species model to species model. In humans, the δ-opioid receptor is most heavily expressed in the basal ganglia and neocortical regions of the brain.
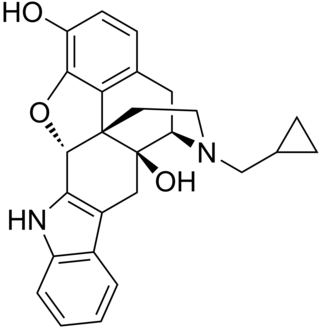
Naltrindole is a highly potent, highly selective delta opioid receptor antagonist used in biomedical research. In May 2012 a paper was published in Nature with the structure of naltrindole in complex with the mouse δ-opioid G-protein coupled receptor, solved by X-ray crystallography.

Tachykinin receptor 3, also known as TACR3, is a protein which in humans is encoded by the TACR3 gene.

Dopamine receptor D3 is a protein that in humans is encoded by the DRD3 gene.

Guanine nucleotide-binding protein G(o) subunit alpha is a protein that in humans is encoded by the GNAO1 gene.

Azaprocin is a drug which is an opioid analgesic with approximately ten times the potency of morphine, and a fast onset and short duration of action. It was discovered in 1963, but has never been marketed.

Alazocine, also known more commonly as N-allylnormetazocine (NANM), is a synthetic opioid analgesic of the benzomorphan family related to metazocine, which was never marketed. In addition to its opioid activity, the drug is a sigma receptor agonist, and has been used widely in scientific research in studies of this receptor. Alazocine is described as a potent analgesic, psychotomimetic or hallucinogen, and opioid antagonist. Moreover, one of its enantiomers was the first compound that was found to selectively label the σ1 receptor, and led to the discovery and characterization of the receptor.

Salvinorin B methoxymethyl ether is a semi-synthetic analogue of the natural product salvinorin A used in scientific research. It has a longer duration of action of around 2–3 hours, compared to less than 30 minutes for salvinorin A, and has increased affinity and potency at the κ-opioid receptor. It is prepared from salvinorin B. The crystal structure is almost superimposable with that of salvinorin A. Structures bound to the κ-opioid receptor have also been reported.
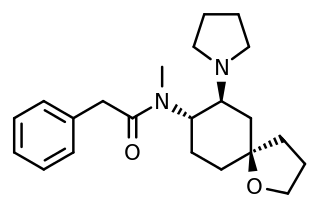
U-69,593 is a drug which acts as a potent and selective κ1-opioid receptor agonist. In animal studies it has been shown to produce antinociception, anti-inflammation, anxiolysis, respiratory depression, and diuresis, while having little effect on gastrointestinal motility. It also inhibits the peripheral, though not central secretion of oxytocin and vasopressin in rats.

IBNtxA, or 3-iodobenzoyl naltrexamine, is an atypical opioid analgesic drug derived from naltrexone. In animal studies it produces potent analgesic effects that are blocked by levallorphan and so appear to be μ-opioid mediated, but it fails to produce constipation or respiratory depression, and is neither rewarding or aversive in conditioned place preference protocols. These unusual properties are thought to result from agonist action at a splice variant or heterodimer of the μ-opioid receptor, rather than at the classical full length form targeted by conventional opioid drugs.
Leumorphin, also known as dynorphin B1–29, is a naturally occurring endogenous opioid peptide. Derived as a proteolytic cleavage product of residues 226-254 of prodynorphin, leumorphin is a nonacosapeptide and has the sequence Tyr-Gly-Gly-Phe-Leu-Arg-Arg-Gln-Phe-Lys-Val-Val-Thr-Arg-Ser-Gln-Glu-Asp-Pro-Asn-Ala-Tyr-Ser-Gly-Glu-Leu-Phe-Asp-Ala. It can be further reduced to dynorphin B and dynorphin B-14 by pitrilysin metallopeptidase 1, an enzyme of the endopeptidase family. Leumorphin behaves as a potent and selective κ-opioid receptor agonist, similarly to other endogenous opioid peptide derivatives of prodynorphin.
Deltorphin, also known as deltorphin A and dermenkephalin, is a naturally occurring, exogenous opioid heptapeptide and thus, exorphin, with the amino acid sequence Tyr-D-Met-Phe-His-Leu-Met-Asp-NH2. Along with the other deltorphins (such as deltorphin I and deltorphin II) and the dermorphins, deltorphin is endogenous to frogs of the genus Phyllomedusa such as P. bicolor and P. sauvagei where it is produced in their skin, and is not known to occur naturally in any other species. Deltorphin is one of the highest affinity and most selective naturally occurring opioid peptides known, acting as a very potent and highly specific agonist of the δ-opioid receptor.


















