Nonsteroidal anti-inflammatory drugs (NSAIDs) are members of a drug class that reduces pain, decreases fever, prevents blood clots, and in higher doses, decreases inflammation. Side effects depend on the specific drug but largely include an increased risk of gastrointestinal ulcers and bleeds, heart attack, and kidney disease.
In chemistry, a zwitterion, also called an inner salt, is a molecule that contains an equal number of positively- and negatively-charged functional groups. With amino acids, for example, in solution a chemical equilibrium will be established between the "parent" molecule and the zwitterion.

Acridine is an organic compound and a nitrogen heterocycle with the formula C13H9N. Acridines are substituted derivatives of the parent ring. It is a planar molecule that is structurally related to anthracene with one of the central CH groups replaced by nitrogen. Like the related molecules pyridine and quinoline, acridine is mildly basic. It is an almost colorless solid, which crystallizes in needles. There are few commercial applications of acridines, at one time acridine dyes were popular but they are now relegated to niche applications, such as with acridine orange. The name is a reference to the acrid odour and acrid skin-irritating effect of the compound.
Mefenamic acid is a member of the anthranilic acid derivatives class of nonsteroidal anti-inflammatory drugs (NSAIDs), and is used to treat mild to moderate pain. It is not widely used in the United States due to its side effects and high cost compared to other NSAIDs.
The Hofmann rearrangement is the organic reaction of a primary amide to a primary amine with one fewer carbon atom. The reaction involves oxidation of the nitrogen followed by rearrangement of the carbonyl and nitrogen to give an isocyanate intermediate. The reaction can form a wide range of products, including alkyl and aryl amines.
In the Ullmann condensation or Ullmann-type reaction is the copper-promoted conversion of aryl halides to aryl ethers, aryl thioethers, aryl nitriles, and aryl amines. These reactions are examples of cross-coupling reactions.
Aromatic sulfonation is an organic reaction in which a hydrogen atom on an arene is replaced by a sulfonic acid functional group in an electrophilic aromatic substitution. Aryl sulfonic acids are used as detergents, dye, and drugs.

Sulindac is a nonsteroidal anti-inflammatory drug (NSAID) of the arylalkanoic acid class that is marketed as Clinoril. Imbaral is another name for this drug. Its name is derived from sul(finyl)+ ind(ene)+ ac(etic acid) It was patented in 1969 and approved for medical use in 1976.

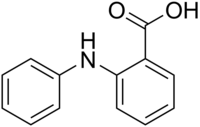
Anthranilic acid is an aromatic acid with the formula C6H4(NH2)(CO2H) and has a sweetish taste. The molecule consists of a benzene ring, ortho-substituted with a carboxylic acid and an amine. As a result of containing both acidic and basic functional groups, the compound is amphoteric. Anthranilic acid is a white solid when pure, although commercial samples may appear yellow. The anion [C6H4(NH2)(CO2)]−, obtained by the deprotonation of anthranilic acid, is called anthranilate. Anthranilic acid was once thought to be a vitamin and was referred to as vitamin L1 in that context, but it is now known to be non-essential in human nutrition.

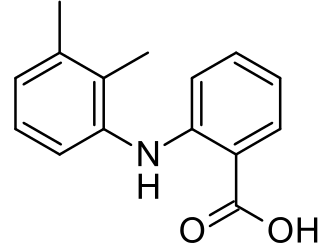
Tolfenamic acid is a member of the anthranilic acid derivatives class of NSAID drugs discovered by scientists at Medica Pharmaceutical Company in Finland. Like other members of the class, it is a COX inhibitor and prevents formation of prostaglandins.
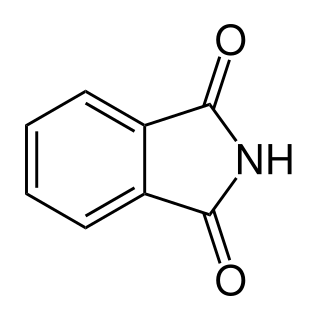
Phthalimide is the organic compound with the formula C6H4(CO)2NH. It is the imide derivative of phthalic anhydride. It is a sublimable white solid that is slightly soluble in water but more so upon addition of base. It is used as a precursor to other organic compounds as a masked source of ammonia.

Meclofenamic acid is a drug used for joint, muscular pain, arthritis and dysmenorrhea. It is a member of the anthranilic acid derivatives class of nonsteroidal anti-inflammatory drugs (NSAIDs) and was approved by the US FDA in 1980. Like other members of the class, it is a cyclooxygenase (COX) inhibitor, preventing the formation of prostaglandins.
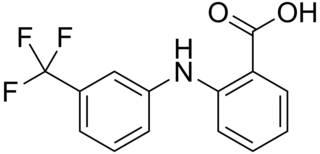
Flufenamic acid (FFA) is a member of the anthranilic acid derivatives class of nonsteroidal anti-inflammatory drugs (NSAIDs). Like other members of the class, it is a cyclooxygenase (COX) inhibitor, preventing the formation of prostaglandins. FFA is known to bind to and reduce the activity of prostaglandin F synthase and activate TRPC6.

Acridone is an organic compound based on the acridine skeleton, with a carbonyl group at the 9 position. A yellow solid.

N-Acetylanthranilic acid is an organic compound with the molecular formula C9H9NO3. It is an intermediate product in catabolism of quinaldine in Arthrobacter sp., and is further metabolized to anthranilic acid.

Meseclazone (W-2395), also known as 2-methylseclazone, is a nonsteroidal anti-inflammatory drug (NSAID) developed in the 1970s. It functions as a prodrug to the 5-chloro derivative of salicyclic acid. It was never marketed on account of toxicity issues, namely pertaining to the liver.

Nifurquinazol (NF-1088) is an antibacterial agent of the nitrofuran class. It was never marketed.
Thiosalicylic acid is an organosulfur compound containing carboxyl and sulfhydryl functional groups. Its molecular formula is C6H4(SH)(CO2H). it is a yellow solid that is slightly soluble in water, ethanol and diethyl ether, and alkanes, but more soluble in DMSO.

2-Iodobenzoic acid, or o-iodobenzoic acid, is an organic compound with the formula IC6H4COOH. The synthesis of 2-iodobenzoic acid via the diazotization of anthranilic acid is commonly performed in university organic chemistry labs. One of its most common uses is as a precursor for the preparation of IBX and Dess–Martin periodinane, both used as mild oxidants.

Isatoic anhydride is an organic compound derived from anthranilic acid. A white solid, it is prepared by reaction of anthranilic acid with phosgene.