 | |
| Clinical data | |
|---|---|
| Trade names | Tandearil, Tanderil |
| AHFS/Drugs.com | International Drug Names |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.004.489 |
| Chemical and physical data | |
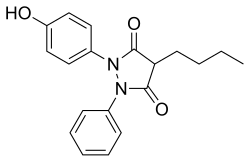
| Formula | C19H20N2O3 |
| Molar mass | 324.380 g·mol−1 |
| 3D model (JSmol) | |
| Chirality | Racemic mixture |
| |
| |
| | |
Oxyphenbutazone is a nonsteroidal anti-inflammatory drug (NSAID). [1] It is a metabolite of phenylbutazone. [2]
It was withdrawn from markets worldwide in the mid-1980s due to bone marrow suppression and the risk of Stevens–Johnson syndrome. [3] [4]